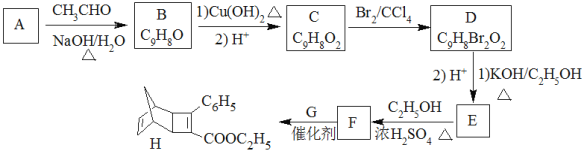
��Ŀ����
����Ŀ������ͭ����(CuSO4��xH2O)��һ����;�㷺���Լ���ijС����̽������ͭ���ʲ��ⶨ�侧���нᾧˮ������
ʵ��(һ)��̽������ͭ�������ԡ�
ȡ��������ͭ��Һ���Թ�,����(NH4)2SO3��Һ,��������M�����ˡ�ϴ�ӡ���M���塣Ϊ��̽��M����ɣ���������ʵ�飺
��ȡһ����M����ֳ�����;
����һ�ݹ����м���ϡ����,�����̼�����ζ������X,������Xͨ����ˮ��,��ˮ��ɫ;��Һ�����ɫ���к�ɫ�������ɡ�
������һ�ݹ����м���Ũ�ռ���Һ,����,����������Yͨ�������Һ��,��Һ���ɫ��
(1)X�Ļ�ѧʽ��______________��
(2)���ⶨ,M�������ӡ������Ӹ���֮��Ϊ2��1��д��M��ϡ���ᷴӦ�Ļ�ѧ����ʽ��____________��
ʵ��(��)��̽������ͭ��������ȶ��ԡ�
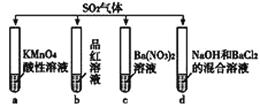
����ȡ��������ͭ�������ʵ��,װ����ͼ��ʾ��
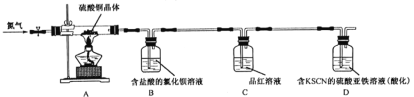
�۲쵽��ʵ������A����ɫ������ɰ�ɫ��ĩ,����ɺ�ɫ��ĩ;B�в�����ɫ����;D����ɫ��Һ���ɫ��Һ��
(3)B���������ữ��Ŀ����______________��C��������__________________________________��
(4)D���е��ʲμӷ�Ӧ�����ӷ���ʽ��________________________________________________��
ʵ��(��)���ⶨ����ͭ�����нᾧˮ������
ȡwg����ͭ����(CuSO4��xH2O)���Ƴ�250mL��Һ,ȡ20.00mL��������Һ��cmol��L-1EDTA(��ΪNa4Y)��Һ�ζ����յ�,����EDTA�ζ�Һ�ݻ�ΪVmL��(�ζ���Ӧ��Cu2++Y4-=CuY2-)
(5)x=_____________________(�ô���ʽ��ʾ)��
(6)���������ʹ���xֵƫ�����______________(����ĸ)��
a����Ʒʧȥ���ֽᾧˮ b���ζ���δ��EDTA��Һ��ϴ
c����ʼ����ʱ�ζ��ܼ���������(�յ�ʱ����������) d���ζ��յ�ʱ���ӿ̶���
���𰸡�SO22NH4CuSO3+2H2SO4=CuSO4+(NH4)2SO4+2SO2��+2H2O+Cu������SO2��ˮ��Ӧ��ɫ��Һ����ɫ��Һ4Fe2-+O2+4H+=4Fe3++2H2O(40w��80cV)/9Vcd
��������
��1����X�����ʿɵ�XΪSO2���ʴ�Ϊ��SO2����2��M�м���Ũ�ռ���Һ�����ȣ���������Y����������ʹʪ��ĺ�ɫʯ����ֽ������˵������YΪNH3��M�к���笠����ӣ�M��ϡ���ᷴӦʱ����Һ�����ɫ���к�ɫ�������ɣ�˵��M�к���Cu+��Cu+����������������Cu��Cu2���������д̼�����ζ������SO2����M�к���SO32����HSO3��������M�������ӡ������Ӹ���֮��Ϊ2:1����M�к���SO32����M�Ļ�ѧʽΪ��NH4CuSO3���������ᷴӦ�ķ���ʽΪ��2NH4CuSO3+2H2SO4=CuSO4+(NH4)2SO4+2SO2��+2H2O+Cu������3�������������ˮ�е��ܽ�Ƚϴ���һ���������ᣬ������Һ�������ӵ�Ũ�ȣ����ʵ�����SO2��ˮ��Ӧ�������SO2����C��ʹƷ����ɫ���ʴ�Ϊ������SO2��ˮ��Ӧ����ɫ��Һ����ɫ��Һ����4�������������£�����ͭ����ֽ����ɵ�������Fe2+������Fe3+��Fe3+����SCN����Ӧ����Fe(SCN)3����һ����Ӧ�е��������μӣ��ʴ�Ϊ��4Fe2++O2+4H+=4Fe3++2H2O����5���ɵζ�����ʽCu2++ Y4��=CuY2����֪��n(CuSO4)=V��10��3L��c mol/L��250ml��20ml=![]() mol����n(H2O)=
mol����n(H2O)=![]() mol������x=
mol������x= ![]() =(40w��80cV)/9Vc���ʴ�Ϊ��(40w��80cV)/9Vc����6��a.��Ʒʧȥ���ֽᾧˮ����ʹ�ⶨ�Ľᾧˮ����ƫ�٣�xƫС����a������b. �ζ���δ��EDTA��Һ��ϴ����ʹ���ĵı�Һ���ƫ�ⶨ�Ľᾧˮ����ƫС��xƫС����b������c.��ʼ����ʱ�ζ��ܼ��������ݶ��յ�ʱ�����ݣ���ʹ��ȡ�ı�Һ���ƫ�ⶨ�Ľᾧˮ����ƫ�٣�xƫС����c������d.�ζ���ʼʱƽ�ӡ��ζ��յ�ʱ���ӣ���ʹ��ȡ�ı�Һ���ƫС���ⶨ�Ľᾧˮ����ƫ��xƫ��d��ȷ����ѡd��
=(40w��80cV)/9Vc���ʴ�Ϊ��(40w��80cV)/9Vc����6��a.��Ʒʧȥ���ֽᾧˮ����ʹ�ⶨ�Ľᾧˮ����ƫ�٣�xƫС����a������b. �ζ���δ��EDTA��Һ��ϴ����ʹ���ĵı�Һ���ƫ�ⶨ�Ľᾧˮ����ƫС��xƫС����b������c.��ʼ����ʱ�ζ��ܼ��������ݶ��յ�ʱ�����ݣ���ʹ��ȡ�ı�Һ���ƫ�ⶨ�Ľᾧˮ����ƫ�٣�xƫС����c������d.�ζ���ʼʱƽ�ӡ��ζ��յ�ʱ���ӣ���ʹ��ȡ�ı�Һ���ƫС���ⶨ�Ľᾧˮ����ƫ��xƫ��d��ȷ����ѡd��

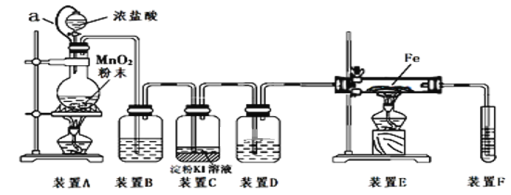
����Ŀ��Ŀǰ������ʹ����㷺�Ľ���֮һ����֪�ڸ����£�Fe��ˮ�����ɷ�����Ӧ��
Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ�������Fe��ˮ�����ķ�Ӧʵ������
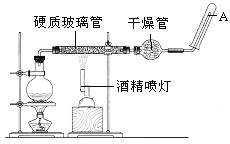
��ش����е����⡣
��1���������ڱ��е�λ����_______
��2����д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������������������ﷴӦԭ�������Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ
��4���������Ͳ���ɵĺϽ�a mol������Pt�����ʵ�������Ϊx���гɷ�ĩ״��ȫ��Ͷ�뺬bmol HNO3��ϡ��Һ�У�ʹ���ַ�Ӧ����HNO3�Ļ�ԭ����ֻ��NO���Իش��������⣺
����HNO3���������ӣ���Һ�еĽ������ӺͲ�������ijɷ�����������������������ӷ�����д�±��հף�
�� | �� | �� | �� | |
��Һ�еĽ������� | Fe2+ | |||
��������ɷ� | Fe��Pt | Pt | Pt |
����x="0.5" ������Һ��Fe3+��Fe2+�����ʵ�����ȣ��ڱ�״���¹�����112mLNO��
��a = ��b = ��
����Ŀ��̼Ԫ�������ǵ��ճ��������Ϳ�ѧ�о��ܲ��ɷ֡���ش��������⡣
(1)��ϩ����ˮ������Ĺ�Ч,��ϩ�ĵ���ʽ��______________��
(2)Al2O3���̼�Ȼ�ԭһ�Ȼ�����һ���µ���������,�÷����̶�,�豸��,����Ŀǰ�����ᳫ���ܼ��š����������Ĵ���
������ұ�������з����ķ�Ӧ�У�
(��)2Al2O3(s)+9C(s)=Al4C3(s)+6CO(g) ��H1;
(��)Al2O3(s)+Al4C3(s)+3AlCl3(g)=9AlCl(g)+3CO(g) ��H2;
(��)3AlCl(g)=AlCl3(g)+2Al(l) ��H3;
��Al2O3(s)+3C(s)= 2Al(l)+ 3CO(g) ��H4=___________________(�ú���H1����H2����H3�Ĵ���ʽ��ʾ)��
��Al4C3�����̼�Ȼ�ԭһ�Ȼ����������м�����������ˮ��Ӧ��������л���÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________��
(3)���û���̿�Ļ�ԭ�Կɴ�����������β��(��������),�������·�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H��0��һ��������,�ܱ������е��й����ʵ�Ũ����ʱ��ı仯���±���ʾ��
N2(g)+CO2(g) ��H��0��һ��������,�ܱ������е��й����ʵ�Ũ����ʱ��ı仯���±���ʾ��
ʱ��/mim Ũ��/(mol/L) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 2.0 | 1.16 | 0.40 | 0.40 | 0.6 | 0.6 |
N2 | 0 | 0.42 | a | b | 1.2 | 1.2 |
CO2 | 0 | 0.42 | a | b | 1.2 | 1.2 |
��0~20min�ڵ�ƽ����Ӧ����v(CO2)=_______mol��L-1��min-1����һ�δﵽƽ���ƽ�ⳣ��K=__________��
��30minʱֻ�ı�ijһ��������ı������������______________ (����ĸ���)��
a�������¶� b�������¶� c����ͨ��һ������NO
d����С��������� e��������ʵĴ��� f�����������ݻ�
(4)����¯�з������ӵĻ�ѧ��Ӧ,���а�����Ӧ��C(s)+CO2(g)![]() 2CO(g)��H>0����1molCO2��������̼���뵽һ����ѹ�ܱ������У���ѹǿΪP�����ﵽƽ��ʱ,��������������������¶ȵĹ�ϵ����ͼ��
2CO(g)��H>0����1molCO2��������̼���뵽һ����ѹ�ܱ������У���ѹǿΪP�����ﵽƽ��ʱ,��������������������¶ȵĹ�ϵ����ͼ��

��CO2�������Ϊ86%ʱ,CO2��ת����Ϊ______________%(�������һλС��,��ͬ)��
����֪�������ѹP��=P������������������ﵽƽ��ʱ������ķ�ѹ��������Ũ������ʾ��ƽ�ⳣ��ΪKp,��900��ʱ,Kp=______________(�ú�p���Ĵ���ʽ��ʾ)��