��Ŀ����
�������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��.�������ƾ�����Ʊ���
��ҵ������CaO2��8H2O����Ҫ�������£�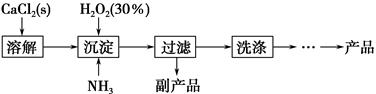
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��_________________________________��
��2������ʱ���ñ�ˮ�����¶���10 �����º�ͨ�������NH3�������ԭ��ֱ��Ǣ�__________________________����_____________________________��
��.�������ƾ��庬���IJⶨ��
ȷ��ȡ0.300 0 g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.020 0 mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
�ζ��յ�۲쵽������Ϊ_______________________________________��
��4�����ݱ������ݣ������Ʒ��CaO2��8H2O������������д��������̣���
KMnO4����Һ�ζ�����
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.300 0 | 1.02 | 24.04 |
| 2 | 0.300 0 | 2.00 | 25.03 |
| 3 | 0.300 0 | 0.20 | 23.24 |
��.��1��CaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl����2�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ����ֹ��������ķֽ⣩����ͨ�����NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣨��ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣬����߲�Ʒ�IJ��ʣ�
��.��3�����������һ��KMnO4����Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ����30 s����ɫ
��4�����ݷ�Ӧ����ʽ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O��CaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl����������ʽ��
5��CaO2��8H2O����5H2O2��2KMnO4
n��CaO2��8H2O���� n��KMnO4����
n��KMnO4���� ��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol
��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol
CaO2��8H2O������������ ��100%��82.91%��
��100%��82.91%��
����

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����װ�û������ʵ��Ŀ�����������
| A���١���ʵ������ȡ���ռ�����NH3 |
| B���ڡ������廯�ء�90%���ᡢ�Ҵ�Ϊԭ�Ϻϳ��������װ�� |
| C���ۡ������װ�������� |
| D���ܡ��������ſ������ռ�CO2 |
��ͼ��ʾװ���У����رջ�������Ʒ����Һ�ޱ仯��ʯ����Һ��죬ʯ��ˮ����ǡ��ݴ��ж�������ƿ��ʢ�ŵ������ǣ� ��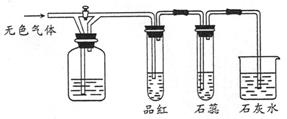
| A��SO2��H2SO4��Ũ�� |
| B��SO2�ͱ���NaHCO3 |
| C��Cl2��NaHSO3 |
| D��NO2��KOH |
��ʵ��������Ũ�����MnO2���������Ʊ�װ����Ӧ��װ��Һ©��������ʹ�ó���©�����й����������������
| A����ֹ������ɢ�������������Ⱦ | B�����ڿ��Ƽ���������� |
| C������©������������Һ�� | D����������HCl�ӷ��������� |
�������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ��������ClO2��һ�ֻ���ɫ�����壬������ˮ��ʵ������NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2���������£�
��1����ȥClO2�е�NH3��ѡ�õ��Լ��� ����������ȡNH3����________������ţ�
| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ E.NH4HCO3 |
��3��д�����ʱ���������ĵ缫��Ӧʽ_
��4���ⶨClO2�Ĺ������£�����ƿ�м��������ĵ⻯�أ���100mLˮ�ܽ���ټ�3mL������Һ���ڲ���Һ����м���ˮ�������ɵ�ClO2����ͨ����������ƿ�б����գ�����������е�ˮ��Һ������ƿ�У����뼸�ε�����Һ����cmol/L��������Ʊ���Һ�ζ� (I2+2S2O32��=2I��+S4O62��)������ȥVmL�����������Һ��
����д��������������������⻯����Һ��Ӧ�����ӷ���ʽ
�ڲ��ClO2������m(ClO2)= �����ú�c��V�Ĵ���ʽ��ʾ��
Ϊ�˾������ռ�������ʹ���ʯ�Ƶõ�CO2���壬����ͼ��ѡ����ʵĵ�װ�ò����ӡ���������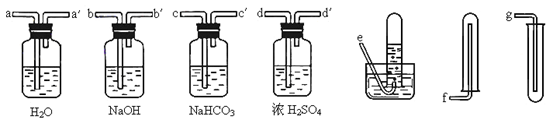
| A��a-a���d-d���e | B��b-b���d-d���g |
| C��c-c���d-d���g | D��d-d���c-c���f |


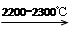 CaC2��CO
CaC2��CO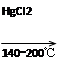 CH2=CHCl
CH2=CHCl