��Ŀ����
�����12�֣�
����������ϩ������Ҫ�Ļ�����Ʒ�����������107t���ҡ�������ʵ�����Ʊ�������ϩ�Ĺ�ҵ�������ж��ֲ�ͬ������
���������գ�
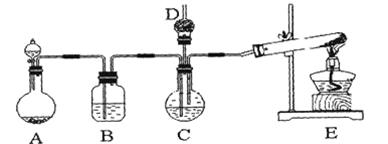
(1)ʵ������ȡ���������������˶������̡�Ũ�����Ũ���ᣬ����Ҫ_________��________����д�Լ�����Һ���ƣ�
(2)ʵ������2.00mol/L�������Ư�۾�[�ɷ�ΪCa(ClO)2��CaCl2]��Ӧ�����������Ȼ��ƺ�ˮ��������2.24L����״����������������Ӧ������Ϊ_________m,l��
(3)ʵ����ͨ���������ſ������ռ����������һ����ʵ�飬��֤���ռ����������Ƿ��п�����
_________________
4)��ҵ���õ�ʯ����Ȳ��������ϩ�ķ�Ӧ���£�
CaO��3C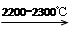 CaC2��CO
CaC2��CO
CaC2��2H2O��HC��CH����Ca(OH)2
HC��CH����HCl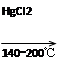 CH2=CHCl
CH2=CHCl
��ʯ����Ȳ�����ŵ������̼���Ʒ���ȸߣ����Ҳ�������ʯ����Դ��
��ʯ����Ȳ����ȱ����___________________��_____________________��
(5)�����������Ӧ���Ƶ�ClCH2CH2Cl��ClCH2CH2Cl���ȷֽ�õ�����ϩ���Ȼ��⡣���һ������ϩ������Ϊԭ����ȡ����ϩ�ķ���������ԭ����ѡ�����û�ѧ����ʽ��ʾ������ע����Ӧ��������
Ҫ�ٷ�Ӧ�������Ȼ��������������ϩ���Ʊ����ڲ��ٲ���������Һ��
___________________________________
(1)�����Ȼ�����Һ������������Һ (2)100
(3)���Թ��ռ��������ռ������Թܵ���������������Һ�У��۲��Թ������������塣
(4)���ܺ� ����Ⱦ����
(5)CH2=CH2��Cl2��ClCH2CH2Cl ClCH2CH2Cl��CH2=CHCl��HCl HC��CH��HCl��CH2=CHCl
�������������(1)Ũ�����ӷ������ɵ������к����Ȼ��⣬Ӧ���ñ����Ȼ�����Һ��ȥ�������ж���Ҫβ��������������������Һ���ա�
(2)���ݷ�Ӧ����������֪����Ӧ�Ļ�ѧ����ʽΪ4HCl��Ca(ClO)2��CaCl2��2H2O��2Cl2������Ӧ���������������ʵ�����0.1mol���������Ȼ�������ʵ�����0.2mol�������Ũ����2.00mol/L�������Ҫ�������Һ�������100ml��
(3)������������������Һ��ȫ��Ӧ�����������������Ʋ���Ӧ���ݴ˼��飬���Թ��ռ��������ռ������Թܵ���������������Һ�У��۲��Թ������������塣
(4)�������̿�֪����Ӧ��Ҫ���£����ȱ��֮һ�Ǹ��ܺģ���Ҫ�Ȼ����������������ؽ��������ȱ��֮���ǻ���Ⱦ������
(5)�����������Ӧ���Ƶ�ClCH2CH2Cl��ClCH2CH2Cl���ȷֽ�õ�����ϩ���Ȼ��⣬�������Ȼ����������Ȳ��Ӧ����������ϩ����˷���ΪCH2=CH2��Cl2��ClCH2CH2Cl��ClCH2CH2Cl��CH2=CHCl��HCl��HC��CH��HCl��CH2=CHCl��
���㣺���������Ʊ������������㡢�����Ʊ���������Լ����۵�

 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д��������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��.�������ƾ�����Ʊ���
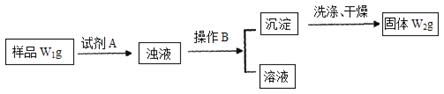
��ҵ������CaO2��8H2O����Ҫ�������£�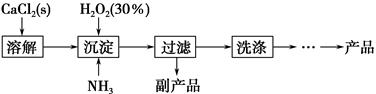
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��_________________________________��
��2������ʱ���ñ�ˮ�����¶���10 �����º�ͨ�������NH3�������ԭ��ֱ��Ǣ�__________________________����_____________________________��
��.�������ƾ��庬���IJⶨ��
ȷ��ȡ0.300 0 g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.020 0 mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
�ζ��յ�۲쵽������Ϊ_______________________________________��
��4�����ݱ������ݣ������Ʒ��CaO2��8H2O������������д��������̣���
KMnO4����Һ�ζ�����
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.300 0 | 1.02 | 24.04 |
| 2 | 0.300 0 | 2.00 | 25.03 |
| 3 | 0.300 0 | 0.20 | 23.24 |
����ɫ������A������ɫ���ߵ�ѹ���������ص㣬�����������˵绯ѧ��ĸ߶����ӡ��ڳ��º���������£�������A�����ȶ��Ĵ��ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������ͬʱ����һ�����嵥�ʡ�ij��ȤС���ͬѧ�Ի�����A������ɷ�����ȷ��A�н�����O��K��Fe����Ԫ�ء�ȡ3.96g������A�ķ�ĩ����ˮ���μ�������ϡ���ᣬ��Ӧ�����Һ�м��뺬��0.08mol KOH����Һ��ǡ����ȫ��Ӧ�����ˣ���ϴ�Ӻ�ij���������գ��õ�����ɫ�����ĩ1.60g����������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ����10.44g��
��1��������A�Ļ�ѧʽΪ ��������A��H2O��Ӧ�����ӷ���ʽΪ ��
��2��������A������Ϊһ�֡���ɫ��Ч��ܡ�ˮ��������ԭ���� ��
��3��������A���Ʊ�����ͨ������������д����KOH�����������ô�������������������Ʊ�A�Ļ�ѧ����ʽ ��
��4��Ŀǰ��������Ի�����A���ȶ��Խ����˴�����̽������ȡ����һ���Ľ�չ�������������п����������Aˮ��Һ�ȶ��Ե���
| A���������� | B��KOH | C������ | D��Fe(NO3)3 |
 I2+I-��2S2O32-+I2=2I- + S4O62-)
I2+I-��2S2O32-+I2=2I- + S4O62-)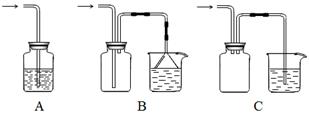
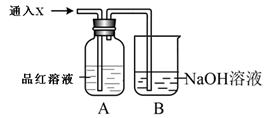
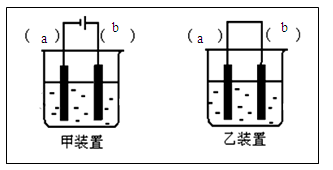


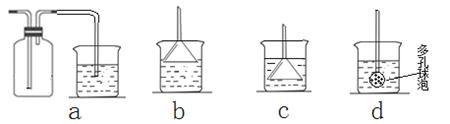
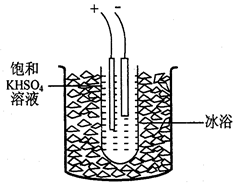
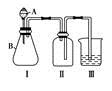 (4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
(4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
 N2O3(g)����ƽ�ⳣ������ʽΪK = ��
N2O3(g)����ƽ�ⳣ������ʽΪK = ��