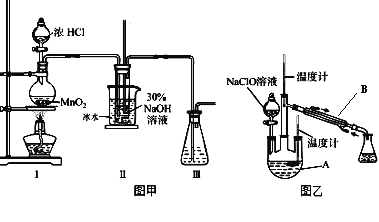
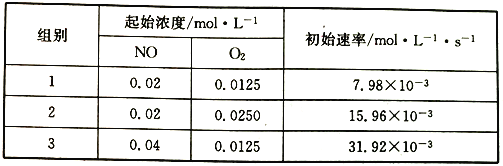
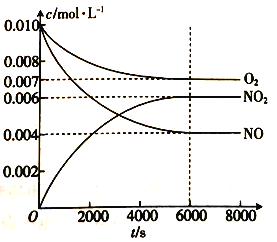
题目内容
【题目】一种从废钴锰催化剂[含53.1%(CH3COO)2Co、13.2%(CH3COO)2Mn、23.8%CoCO3、6.5%Mn(OH)2、1.3%SO2及对二甲苯等有机物等]中回收钴和锰的工艺流程如下:
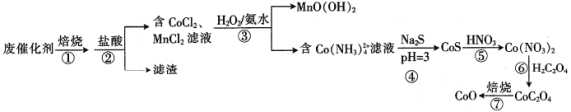
回答下列问题:
(1)步骤①焙烧的目的是_________________________________。
(2)步骤②酸浸时,控制盐酸适当过量、溶液加热并充分搅拌,其目的是___________。
(3)步骤③MnCl2与H2O2和氨水反应的离子方程式为______________________。
(4)步骤④调节pH时采用CH3COOH和CH3 COONa混合溶液,该混合溶液称为缓冲溶液,该溶液中加入少量的酸、碱或稀释时pH变化均不大,其中稀释时pH变化不大的原因是______________________。
(5)步骤⑤硝酸溶解CoS生成Co(NO3)2同时生成NO和S,该反应的化学方程式为______________________。
(6)步骤⑦若在实验室进行,所需的硅酸盐质仪器除酒精灯和玻璃棒外,还有___________(填仪器名称)。
(7)某工厂用mkg废催化剂最终制得 CoO n kg,则CoO的产率为___________。
【答案】将二甲苯等有机物氧化除去,并将(CH3COO)2Co、(CH3COO)2Mn转化为无机物 提高钴、锰的浸出率 Mn2++H2O2+2NH3·H2O=MnO(OH)2↓+2NH4++H2O 稀释时,CH3COOH电离度增大产生的H+和CH3COO-水解程度增大产生的OH-几乎抵消 3CoS+8HNO3=3Co(NO3)2+3S+2NO↑+4H2O 坩埚、泥三角 ![]()
【解析】
(1)废钻锰催化剂对二甲苯等有机物等受热后挥发或分解。
(2)步骤②酸浸时,控制盐酸适当过量、溶液加热并充分搅拌,使反应更充分。
(3)步骤③MnCl2与H2O2和氨水反应生成MnO(OH)2和铵盐;
(4)缓冲溶液稀释时pH变化均不大,稀释时,CH3COOH电离度增大产生的H+和CH3COO-水解程度增大结合的H+几乎抵消。
(5)步骤⑤硝酸溶解CoS生成Co(NO)2同时生成NO和S,根据电子得失守恒,质量守恒写出方程式。
(6)固体加热分解用坩埚、泥三角、酒精灯和玻璃棒;
(7)产率=实际产量/理论产率。
(1)焙烧可以将废钻锰催化剂中对二甲苯等有机物等氧化除去,并将(CH3COO)2Co、(CH3COO)2Mn转化为无机物。
(2)步骤②酸浸时,控制盐酸适当过量、溶液加热并充分搅拌,使反应更充分,从而提高钴、锰的浸出率。
(3)步骤③MnCl2与H2O2和氨水反应生成MnO(OH)2和铵盐,离子方程式为Mn2++H2O2+2NH3·H2O=MnO(OH)2↓+2NH4++H2O;
(4)缓冲溶液稀释时pH变化均不大,稀释时,CH3COOH电离度增大产生的H+和CH3COO-水解程度增大结合的H+几乎抵消。
(5)步骤⑤硝酸溶解CoS生成Co(NO)2同时生成NO和S,反应的化学方程式为:3CoS+8HNO3=3CO(NO3)2+3S+2NO↑+4H2O;
(6)固体加热分解用坩埚、泥三角、酒精灯和玻璃棒,所需的硅酸盐质仪器除酒精灯和玻璃棒外,还要坩埚、泥三角;
(7)mkg废催化剂中:53.1%(CH3COO)2Co生成CoO:mkg×53.1%×75/177=0.225mkg,23.8%CoCO3生成CoO:mkg×23.8%×75/119=0.15mkg,产率=实际产量/理论产率=n/(0.225+0.15)m ×100%=![]() .
.

 轻松课堂单元期中期末专题冲刺100分系列答案
轻松课堂单元期中期末专题冲刺100分系列答案【题目】甲醇是重要的化工原料,又是一种可再生能源,具有广泛的开发和应用前景。
(1)已知反应CO(g)+2H2(g)![]() CH3OH(g) H=-99kJmol-1中的相关化学键键能如下:
CH3OH(g) H=-99kJmol-1中的相关化学键键能如下:
则x=___________。
化学键 | H-H | C-O | C三O | H-O | C-H |
E/(kJmol-1) | 436 | 343 | x | 465 | 413 |
(2)在一容积可变的密闭容器中,1molCO与2molH2发生反应:CO(g)+2H2(g)![]() CH3OH(g) H1<0,CO在不同温度下的平衡转化率(α)与压强的关系如下图所示。
CH3OH(g) H1<0,CO在不同温度下的平衡转化率(α)与压强的关系如下图所示。
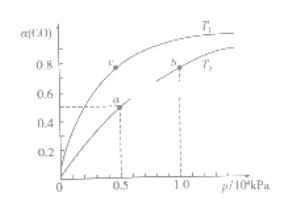
①T1_________T2(填“>”、“<”、“=”);a、b两点的反应速率:v(a)_____v(b)(填“>”、“<”、“=”);
在c点条件下,下列叙述能说明上述反应能达到化学平衡状态的是_____(填字母);
a.H2的消耗速率是CH3OH生成速率的2倍 b.CH3OH的体积分数不再改变
C.混合气体的密度不再改变 d.CO和CH3OH的物质的量之和保持不变
②计算图中a点的平衡常数KP=_____(用平衡分压代替平衡浓度计算,分压=总压×物质的量分数)。
(3)利用合成气(主要成分为CO、CO2和H2)合成甲醇,发生的主要反应如下:
a:CO(g)+2H2(g)![]() CH3OH(g) H1
CH3OH(g) H1
b:CO2(g)+H2(g)![]() CO(g)+H2O(g) H2
CO(g)+H2O(g) H2
c:CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) H3
CH3OH(g)+H2O(g) H3
①述反应对应的平衡常数分别为K1、K2、K3,它们随温度变化的曲线如下图所示。
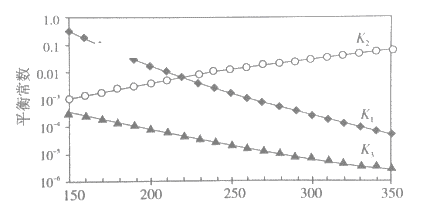
则H1__________H3(填“>”、“<”、“=”),理由是_______。
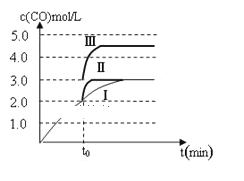
②一定温度下,在3 L容积可变的密闭容器中发生反应b,已知c(CO)与反应时间t变化曲线Ⅰ如图所示,若在t0时刻分别改变一个条件,曲线Ⅰ变为曲线Ⅱ和曲线Ⅲ。当曲线Ⅰ变为曲线Ⅱ时,改变的条件是________;当曲线Ⅰ变为曲线Ⅲ时,改变的条件是_________。
③反应c的 △H___0, △S____0(填“>”“=”或“<”)。