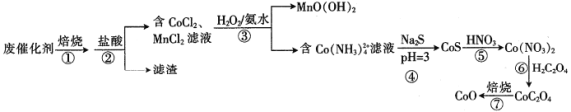
��Ŀ����
����Ŀ��NO��NO2������β������Ҫ�ĺ���������ش��������⣺
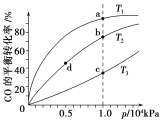
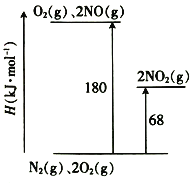
��1����֪��������ת�������е������仯��ͼ(ͼ�б�ʾ����2 mol NO2�������仯)��1 mol NO����ΪNO2���ʱ��H=___________��
��2��ij�¶��£���Ӧ��ƽ�ⳣ�����£�
a.2NO2(g) ![]() N2(g)+2O2(g) K=6.7��1016
N2(g)+2O2(g) K=6.7��1016
b.2NO(g) ![]() N2(g)+O2(g) K=2.2��1030
N2(g)+O2(g) K=2.2��1030
�ֽⷴӦ���ƽϴ�ķ�Ӧ��__________(�a����b��)����Ӧ2NO(g)+O2(g) ![]() 2NO2(g)��K=_____________��
2NO2(g)��K=_____________��
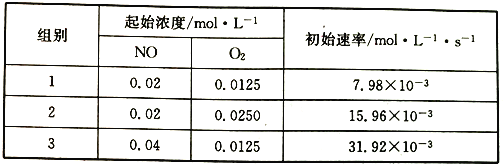
��3����֪��Ӧ2NO(g)+O2(g) ![]() 2NO2������Ӧ����v��=k1Cm(NO)cn(O2)������kΪ���ʳ�������ͨ������ʵ�����ݼ���k��m��n��
2NO2������Ӧ����v��=k1Cm(NO)cn(O2)������kΪ���ʳ�������ͨ������ʵ�����ݼ���k��m��n��
��k1=______________��m=______________��n=______________��
��4����֪�÷�Ӧ������Ϊ��
��һ����NO+NO![]() N2O2 ����ƽ��
N2O2 ����ƽ��
�ڶ�����N2O2+O2![]() 2NO2����Ӧ
2NO2����Ӧ
���пɽ�����Ϊ�ڶ�����Ӧ��Ӱ���һ����ƽ�⣬��һ����Ӧ�У�v(��)=k1c2(NO)��v(��)=k-1c(N2O2)������������ȷ����________(����ĸ)��
A.��һ����Ӧ��ƽ�ⳣ��K=![]()
B.v(��һ��������Ӧ)<v(�ڶ����ķ�Ӧ)
C.�ڶ����Ļ�ܱȵ�һ���Ļ�ܸ�
D.�ڶ�����N2O2��O2����ײ100%��Ч
��5��һ�������²��������NO��O2��NO2Ũ�ȷ������±仯��
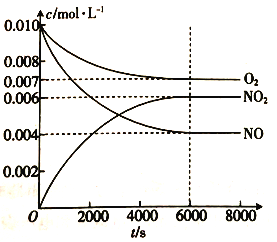
��NO��ƽ��ת����Ϊ _______________��
�ڸ��¶��·�Ӧ2NO(g)+O2(g)![]() 2NO2(g)��ƽ�ⳣ��Ϊ______________(��������)
2NO2(g)��ƽ�ⳣ��Ϊ______________(��������)
���𰸡�-56KJ��mol-1 b 3.28��1013 1596L2��mol-2��s-1 2 1 AC 60% 321
��������
��1����ͼ��ϸ�˹���ɼ��������1molNO2�ų���������
��2��ƽ�ⳣ��Խ��ӦԽ�����У�
��Ӧb-a��2NO(g)+O2(g) ![]() 2NO2(g)��K=2.2��1030/6.7��1016��
2NO2(g)��K=2.2��1030/6.7��1016��
��3������1��2��NOŨ�Ȳ���ʱ��������Ũ������1��������Ҳ����1������n=1��
����1��3��NO��Ũ������1�������ʱ�Ϊԭ����4������m=2����������һ�����ݿ����k1��
��4��A������ƽ�ⳣ���Ķ��������
B��v(��һ��������Ӧ)�ǿ췴Ӧ��
C���ڶ����ķ�Ӧ�ѣ���ܸߣ�
D���ڶ�����N2O2��O2����Ч��ײ��С����Ӧ�ѡ�
��5����NO��ƽ��ת����=NO�ı仯��/NO��Ͷ������
�ڸ��¶��·�Ӧ2NO(g)+O2(g)![]() 2NO2(g)��ƽ�ⳣ��Ϊc2(NO2)/c2(NO)c(O2).
2NO2(g)��ƽ�ⳣ��Ϊc2(NO2)/c2(NO)c(O2).
��1��ͼ�б�ʾ����2 mol NO2�������仯����N2(g)+2O2(g) =2NO2(g) ��H=68KJ��mol-1
��N2(g)+O2(g) =2NO(g) ��H=180KJ��mol-1���ɸ�˹���ɣ���-�ڵ�2NO(g) +O2(g) =2NO2(g) ��H=-112KJ��mol-1������ͬ����2�ã�NO(g) +1/2 O2(g) =NO2(g) ��H=-56KJ��mol-1��
��2��ƽ�ⳣ��Խ��ӦԽ�����У�b.2NO(g) ![]() N2(g)+O2(g)ƽ�ⳣ��K=2.2��1030��ѡb��
N2(g)+O2(g)ƽ�ⳣ��K=2.2��1030��ѡb��
��Ӧb-a��2NO(g)+O2(g) ![]() 2NO2(g)��K=2.2��1030/6.7��1016=3.28��1013��
2NO2(g)��K=2.2��1030/6.7��1016=3.28��1013��
��3������1��2��NOŨ�Ȳ���ʱ��������Ũ������1��������Ҳ����1������n=1��
����1��3��NO��Ũ������1�������ʱ�Ϊԭ����4������m=2��
��m��n�����1�����ݣ�7.98��10-3mol��L��1��s��1=k1����0.02mol��L��1��2��0.0125mol��L��1�����k1=1596L2��mol-2��s-1��
��4��A����һ����Ӧ��ƽ�ⳣ��K=c(N2O2)/c2(NO),ƽ��ʱv(��)=v(��)==k1c2(NO)=k-1c(N2O2)����c(N2O2)/c2(NO)=k1/k-1��K=c(N2O2)/c2(NO)==k1/k-1����A��ȷ��
B��v(��һ��������Ӧ)�ǿ췴Ӧ��v(�ڶ����ķ�Ӧ)������Ӧ��v(��һ��������Ӧ)>v(�ڶ����ķ�Ӧ)����B����
C���ڶ����ķ�Ӧ�ѣ���ܸߣ��ڶ����Ļ�ܱȵ�һ���Ļ�ܸߣ���C��ȷ��
D���ڶ���������Ӧ��˵��N2O2��O2����Ч��ײ�ļ��ʽ�С�������ܴﵽ100%,��D����
��ѡAC��
��5����NO��ƽ��ת����=NO�ı仯��/NO��Ͷ����=��0.010mol��L��1-0.004mol��L��1��/0.010mol��L��1=0.6��
���¶��·�Ӧ2NO(g)+O2(g)![]() 2NO2(g)��ƽ�ⳣ��Ϊc2(NO2)/c2(NO)c(O2)=0.0062/0.0042��0.007=321.
2NO2(g)��ƽ�ⳣ��Ϊc2(NO2)/c2(NO)c(O2)=0.0062/0.0042��0.007=321.

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�