��Ŀ����
13������CO2���ŷ��Լ�CO2����Դ�����þ�����Ҫ���壮��1���ð�ˮ���������е�CO2������Σ��Ǽ���CO2�ŷŵĿ��д�ʩ֮һ��
��д����ˮ���������е�CO2������ε���Ҫ��ѧ����ʽCO2+NH3•H2O�TNH4HCO3��
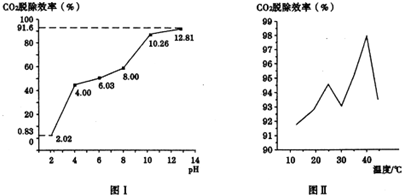
�ڷֱ��ò�ͬpH�����ռ����������е�CO2��CO2�ѳ�Ч�������ռ���pH��ϵ��ͼ����ʾ��CO2�ѳ�Ч�������ռ���pH��ϵ��CO2�ѳ�Ч�������ռ���pH���������������CO2�ĺ���Ϊ12%������ͨ�˰�ˮ������Ϊ0.052m3/h����״��������pHΪ12.81�İ�ˮ��������30min���ѳ���CO2�����ʵ���Ϊ0.13mol����ȷ��0.01����
��CO2�ѳ�Ч�����¶ȵĹ�ϵ��ͼ����ʾ����40�浽45���ѳ�CO2Ч�ʽ��͵���Ҫԭ����̼����������ֽ����ɶ�����̼��
��2����CO2�ͼ��������ƺϳ�����CO��H2����CO2��Դ�����õ���Ч;�����ϳ��������Ʊ��״��ķ�ӦΪ
2H2��g��+CO��g��?CH3OH��g����H=-90.1kJ/mol��
����T��ʱ���ݻ���ͬ�ļס��ҡ������������ܱ������У�����ͬ��ʽͶ�˷�Ӧ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
| ���� | �� | �� | �� | |
| ��ʼ��Ӧ��Ͷ���� | 2mol H2��l mol CO | 1mol CH3OH | 2mol CH3OH | |
| ƽ | c��CH��0H��/mol/L | C1 | C2 | c3 |
| �� | ��Ӧ�������仯ZkJ | x | y | z |
| �� | ��ϵѹǿ/Pa | P1 | P2 | P3 |
| �� | ��Ӧ��ת���� | a1 | a2 | a3 |
A.2c1��c3 B��|x|+|y|��90.1 C.2p2��p3 D��a1+a3��1
����T�桢���������£��÷�Ӧ��ƽ�ⳣ��Kp=6.0��10-3��kPa��-2�����������з�Ӧ�ﵽƽ��ʱp��CH3OH��=24.0kPa����ƽ����������CH3OH�����ʵ�������Ϊ44.4%��Kp����ƽ���ѹ����ƽ��Ũ�ȼ�������ƽ�ⳣ������ѹ=��ѹ�����ʵ�����������
���� ��1���ٰ�ˮ��CO2��Ӧ����̼����泥��ݴ���д��
�ڸ���ͼ1������CO2�ѳ�Ч�������ռ���pH���������������ͨ�˰�ˮ������Ϊ0.052m3/h����״��������pHΪ12.81�İ�ˮ��������30min�����������CO2�ĺ���Ϊ12%�����CO2������
�۸����¶ȸ����ɵ�̼������ֽ����ɶ�����̼������
��2����A���ס�����Ƚϣ��Ѽ�ЧΪ��ʼ����1molCH3OH�����м״������ʵ���Ϊ��2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ���
B������Ŀ��֪����1molCH3OH�������仯Ϊ90.8kJ���ס���ƽ��״̬��ͬ����ƽ��ʱ�״�Ϊnmol�����㷴Ӧ����ֵ���ݴ��жϣ�
C���Ƚ��ҡ�����֪�����м״������ʵ���Ϊ�ҵ�2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ���
D���ס��Ҵ�����ͬ��ƽ��״̬�����1+��2=1����C�ķ�����֪��2����3���ݴ��жϣ�
����2H2��g��+CO��g��?CH3OH��g����ʼ��Ӧ��Ͷ����2mol H2��l mol CO���迪ʼʱ����ѹǿΪ2P��COѹǿΪP��������ʽ������ƽ�ⳣ��Kp=6.0��10-3��kPa��-2�������ƽ��ʱ����ѹ��������CH3OH�����ʵ�������Ϊ$\frac{��ѹ}{��ѹ}$���м��㣮
��� �⣺��1���ٰ�ˮ��CO2��Ӧ����̼����泥���Ӧ�ķ���ʽΪCO2+NH3•H2O�TNH4HCO3���ʴ�Ϊ��CO2+NH3•H2O�TNH4HCO3��
�ڸ���ͼ1������CO2�ѳ�Ч�������ռ���pH�����������Ϊ����ͨ�˰�ˮ������Ϊ0.052m3/h����״����������30minͨ��������к�������̼���Ϊ0.052m3/h��0.5h��12%=0.00312m3�������ʵ���Ϊ$\frac{0.00312��10{\;}^{3}L}{22.4L/mol}$��91.6%=0.13mol���ʴ�Ϊ��CO2�ѳ�Ч�������ռ���pH���������0.13mol��
����Ϊ�¶ȸ����ɵ�̼������ֽ����ɶ�����̼�����Դ�40�浽45���ѳ�CO2Ч�ʽ��ͣ��ʴ�Ϊ��̼����������ֽ����ɶ�����̼��
��2����A���ס�����Ƚϣ��Ѽ�ЧΪ��ʼ����1molCH3OH�����м״������ʵ���Ϊ��2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ�����2c1��c3����A����
B������Ŀ��֪����1molCH3OH�������仯Ϊ90.8kJ���ס���ƽ��״̬��ͬ����ƽ��ʱ�״�Ϊnmol�����ڼ�������a=90.8n������������b=90.8��1-n������a+b=90.8����B����
C���Ƚ��ҡ�����֪�����м״������ʵ���Ϊ�ҵ�2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ�����2p2��p3����C����
D���ס��Ҵ�����ͬ��ƽ��״̬�����1+��2=1����C�ķ�����֪��2����3������a1+a3��1����D��ȷ��
��ѡD��
���� 2H2��g��+CO��g��?CH3OH��g����ʼ��Ӧ��Ͷ����2mol H2��l mol CO���迪ʼʱ����ѹǿΪ2P��COѹǿΪP��
��ʼ��kPa�� 2P P 0
ת����kPa��48.0 24.0 24.0
ƽ�⣨kPa��2P-48 P-24 24
Kp=$\frac{24}{��2P-48��{\;}^{2}����P-24��}$=6.0��10-3��kPa��-2�����P=34.0kPa����ƽ����������CH3OH�����ʵ�������Ϊ$\frac{��ѹ}{��ѹ}$=$\frac{24}{30+24}$��100%=44.4%���ʴ�Ϊ��44.4%��
���� ���⿼�������̼������Ч�ʵ�ʵ��̽������Чƽ�������Լ�ƽ�ⳣ������ؼ���ȣ��ѶȽϴ�ע��ƽ��״̬�������Чƽ�������Ӧ�ã�

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�| A�� | ��ά�ء���֬�������ʾ�����Ȼ�߷��ӻ����� | |
| B�� | ������ϩ������Ʒ������ʳƷ��װ | |
| C�� | ���ع��͡�����ʳ�ã�Ҳ���������Ʒ��� | |
| D�� | ������ʳ��ƾ����˵��ۡ������ǡ��Ҵ��Ļ�ѧ�仯���� |

��������������������ʽ��ʼ�����ͳ�����ȫʱ��pH���±���
| ���� | Fe2+ | Fe3+ | Cu2+ |
| ��ʼ����ʱ��pH����ʼŨ��Ϊ1.0mol/L�� | 6.5 | 1.5 | 4.2 |
| ������ȫʱ��pH | 9.7 | 3.2 | 6.7 |
��1�������¶���п��ˮ��Һ�еĴ�����ʽ��pH�Ĺ�ϵ��ͼ��������Ϊ��Һ��pH��������ΪZn2+��Zn��OH��42-�������ӣ����ʵ���Ũ�ȵĶ���������Zn2+����Ũ��Ϊ10-5mol•L-1ʱ��Zn2+�����ѳ�����ȫ����
����ͼ�����ݼ��㣬Ksp[Zn��OH��2]=1��10-17��
��2��д���Լ�a��b�Ļ�ѧʽ��a��ZnO��Zn��OH��2��b��Zn��
��3��д������H2O2������Ӧ�����ӷ�Ӧ����ʽ��H2O2+2Fe2++2H+�T2Fe3++2H2O��
��4��д����п�����е����ӷ�Ӧ����ʽ��2Zn2++4HCO3-=Zn��OH��2•ZnCO3��+3CO2��+H2O����п����Һ����Ҫ���ʵĻ�ѧʽ�ǣ�NH4��2SO4��
��5������ʽ̼��п���ȷֽ�ɵõ����д����ܵĻ�������п�������ڴ�������״��ϳ�̼���������DMC���ķ�Ӧ��д����ʽ̼��п���ȷֽ�Ļ�ѧ����ʽZn��OH��2•ZnCO3=2ZnO+CO2��+H2O��
��6�������Ȼ�п��Һ��̼������Һ��Ӧ�Ƶõ�̼��п�л��м�ʽ̼��п��ijͬѧȡһ�������ĸù��壬���������أ��ܷ�ͨ�����������ļ�������ȷ������ijɷ֣��� ����ܡ���
| A�� | ��״���£�11.2LSO3������ԭ����Ϊ1.5NA | |
| B�� | ���³�ѹ�£�1.8g H2O�к��еĵ�����Ϊ0.8NA | |
| C�� | ���³�ѹ�£�48g O2��O3�Ļ�����к��е���ԭ����Ϊ3NA | |
| D�� | ��״���£�0.1mol Cl2������NaOH��Һ��Ӧʱ��ת�Ƶĵ�����Ϊ0.2NA |
| A�� | ���ͱ��� | B�� | �����2��2-�������� | ||
| C�� | �Ҵ����Ҷ��� | D�� | 1��1-���������1��2-�������� |
| A�� | �縺�ԣ�X��Y | |
| B�� | ���ڱ��У�X������Y���ұ� | |
| C�� | ��X��Y�γɻ������X�����ۣ�Y�Ը��� | |
| D�� | ��̬�⻯����ȶ��ԣ�HmYǿ��HnX |
| A�� | KBr | B�� | N2 | C�� | HBr | D�� | NaOH |


