��Ŀ����
16��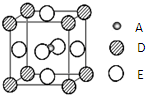 ��֪��A��B��C��D��EΪ���ڱ�1��36���е�Ԫ�أ����ǵ�ԭ������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ��C2-����D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ��E��ԭ������Ϊ28��
��֪��A��B��C��D��EΪ���ڱ�1��36���е�Ԫ�أ����ǵ�ԭ������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ��C2-����D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ��E��ԭ������Ϊ28����ش��������⣺
��1��A��B��C��D����Ԫ���У��縺��������O����Ԫ�ط��ţ���
��2��B���⻯��ķе�Զ����A���⻯�����Ҫԭ����NH3���Ӽ����γ������
��3����A��B��C�γɵ�����CAB-��AC2��Ϊ�ȵ����壬��CAB-��Aԭ�ӵ��ӻ���ʽΪsp��
��4��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2��E2+��������AC�����γ�[E��AC��4]2+����ԭ����AC�����к��й¶Ե��ӣ�
��5��������֣�ֻ��A��D��E����Ԫ�ص�һ�־��壨��������ͼ��ʾ�����г����ԣ��þ���Ļ�ѧʽΪMgNi3C��
���� ����A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȵó�A��1s2 2s2 2p2����AΪ��C��
����C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ����CΪ��O��
����A��B��C��D��Eԭ������������֪BΪ��N��
C2-����D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ����D��Mg��
E��ԭ������Ϊ28����E��Ni��
��1��Ԫ�صķǽ�����Խǿ���縺��Խǿ��
��2��NH3���Ӽ����γ������
��3��CO2��CNO-��Ϊ�ȵ����壬�ȵ�������ӻ���ʽ��ͬ��
��4��E��Ni����Χ������Ϊ10��CO�������й¶Ե��ӣ����γ���λ����
��5����ͼ���Կ���Aԭ�ӵ���λ��Ϊ6���þ���Ļ�ѧʽΪ�����þ�̯�����㣮
��� �⣺����A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȵó�A��1s2 2s2 2p2����AΪ��C��
����C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ����CΪ��O��
����A��B��C��D��Eԭ������������֪BΪ��N��
C2-����D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ����D��Mg��
E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2����E��Ni��
��1��A��B��C��D����Ԫ����O�ķǽ�������ǿ���ǽ�����Խǿ�縺��Խǿ����O�ĵ縺����ǿ���ʴ�Ϊ��O��
��2��A���⻯��ΪCH4 ��B���⻯��ΪNH3��NH3���Ӽ����γ������ʹ���Ӽ���������ǿ���е����ߣ��ʴ�Ϊ��NH3���Ӽ����γ������
��3��CO2��CNO-��Ϊ�ȵ����壬�ȵ�������ӻ���ʽ��ͬ����֪CO2Ϊsp�ӻ�����CNO-Ҳ��sp�ӻ����ʴ�Ϊ��sp��
��4��E��Ni����Χ������Ϊ10��E����Χ�����Ų�ʽΪ3d84s2�����й¶Ե��ӵ����ӻ���ӣ����γ���λ����CO�����к��й¶Ե��ӣ��ʴ�Ϊ��3d84s2���¶Ե��ӣ�
��5����ͼ���Կ���Aԭ�ӵ���λ��Ϊ6���þ���Ļ�ѧʽΪ�����þ�̯�����㣬Cԭ���ھ����ڲ�ԭ����Ϊ��1��Mg�ھ���������ԭ����Ϊ��$\frac{1}{8}$��8=1��Niԭ����������ԭ������Ϊ��$\frac{1}{2}$��6����ѧʽΪ��MgNi3C���ʴ�Ϊ��MgNi3C��
���� ���⿼�������ʽṹ�ƶ��⣬���õ����Ų����й�֪ʶ�����ƶϣ�ͬʱ�������ӻ����ȵ����壬������ѧʽ������ȣ�

| A�� | ����������ȼ�� | B�� | ������������ȼ�� | ||
| C�� | ��������ۻ�ϼ��� | D�� | ͭ�ڿ����м��� |
| A�� | C02��CaO�����Ƿ��ȷ�Ӧ����CaC03�ֽ������ȷ�Ӧ | |
| B�� | ֻ�зֽⷴӦ�������ȷ�Ӧ | |
| C�� | ʹ�ô����ķ�Ӧ�����ȷ�Ӧ | |
| D�� | ������ȵķ�Ӧһ�������ȷ�Ӧ |
| A�� | c��Na+��+c��H+��=c��HRO3-��+2c��RO32-��+c��OH-�� | B�� | c��H+��+c��H2RO3��=c��RO32-��+c��OH-�� | ||
| C�� | c��Na+����c��HRO3-����c��H+����c��OH-����c��RO32-�� | D�� | c��HRO3-����c��H2RO3����c��OH-����c��RO32-����c��H+�� |
| A�� | �����£�10L pH=1��������Һ�к��е�H+��ΪNA | |
| B�� | ��58.5g NaCl����1.00Lˮ�У�����NaCl��Һ��Ũ��Ϊ1.00mol•L-1 | |
| C�� | 1molCl2������������Ӧ��ת�Ƶĵ�����Ϊ3NA | |
| D�� | 1molAl3+���еĺ��������Ϊ3NA |
| A�� | 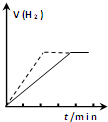 ��һ��������п�������ϡ���ᷴӦ���Թ��м���������CuSO4��Һ����ͼ���߱�ʾ����CuSO4��Һʱ���������ʱ��Ĺ�ϵ | |
| B�� | 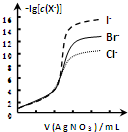 ��0.0100mol/L����������Һ���ζ�Ũ�Ⱦ�Ϊ0.1000mol/LCl-��Br-��I-�Ļ����Һ����ͼ���ߣ���ȷ�����ȳ�������Cl- | |
| C�� | 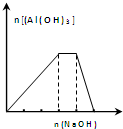 ��NH4Al��SO4��2��Һ�еμӹ�����NaOH��Һ��ͼ�����߱�ʾNaOH�����ʵ�����Al��OH��3���ʵ�����ϵͼ | |
| D�� | 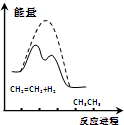 ��ͼ��˵����ϩ��H2�ӳɷ�Ӧ�Ƿ��ȷ�Ӧ�����߱�ʾ���д��������µķ�Ӧ���� |
| A�� | ��ʳ�ɳ�ȥ��ˮ���ڱڵ�ˮ�� | |
| B�� | ���ۣ���֬ �͵����ʶ��Ǹ߷��ӻ����� | |
| C�� | ���ø����������ҩ�ý��һ���˶Խ������Σ�� | |
| D�� | �����ϲ���ʹ�ֻ������Եȵ��Ӳ�Ʒ�����ɡ�ʵ�ú��³� |
| A�� | ˮ�������c��H+��=1.0��10-13mol/L����Һ�У�Na+��NH4+��Cl-��SO42- | |
| B�� | ���������Һ���ɫ����Һ�У�Cl-��AlO2-��HCO3-��NH4+ | |
| C�� | ��������KSCN���Ϊ��ɫ����Һ�У�K+��Mg2+��I-��NO3- | |
| D�� | ��ɫ������Һ�У�CH3COO-��CO32-��K+��Na+ |