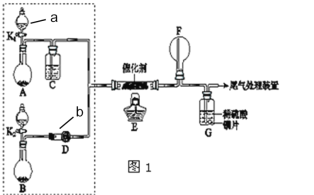
��Ŀ����
����Ŀ������˵����ȷ���ǣ� ��
A.��ij��Һ�еμ�����AgNO3��Һ���а�ɫ������������Һ��һ������Cl-
B.��ij��Һ�м���һ������ϡ���ᣬ����������ʹ����ʯ��ˮ����ǣ���Һ��һ������SO32-��CO32-
C.�����ȵ�ľ̿��Ũ���ᷴӦ���������建��ͨ�����ʯ��ˮ�У���Һû�г�������
D.��ij��Һ�еμ�����ŨNaOH��Һ����ʪ���ɫʯ����ֽ�����Թܿڣ���ֽ��������˵������Һ��һ������NH4+
���𰸡�CD
��������
A����ij��Һ�еμ�AgNO3��Һ�����а�ɫ�����������ų�SO42-��CO32-�����ӵĸ��ţ�Ӧ�ȼ������ữ����A����
B����Һ��Ҳ���ܴ���HCO3-��HSO3-����B����
C. ����ľ̿��Ũ���ᷢ����Ӧ��C+4HNO3(Ũ)![]() CO2��+4NO2��+2H2O�����������ͨ�����ʯ��ˮ���ܷ����ķ�Ӧ�У�Ca(OH)2+CO2=CaCO3��+H2O��3NO2+H2O=2HNO3+NO��CaCO3+2HNO3=Ca(NO3)2+ CO2��+ H2O�����Բ������ɳ�������C��ȷ��
CO2��+4NO2��+2H2O�����������ͨ�����ʯ��ˮ���ܷ����ķ�Ӧ�У�Ca(OH)2+CO2=CaCO3��+H2O��3NO2+H2O=2HNO3+NO��CaCO3+2HNO3=Ca(NO3)2+ CO2��+ H2O�����Բ������ɳ�������C��ȷ��
D. ��ij��Һ�к�NH4+���μ�����ŨNaOH��Һ��������ʹ��ɫʯ����ֽ�����İ�������ֽ����������˵������Һ��һ������NH4+����D��ȷ��
��ѡCD��

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�����Ŀ����1����ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)
FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ�����
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ�����
700�� | 900�� | |
K1 | 1.47 | 2.15 |
K2 | 2.38 | 1.67 |
��ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g)��ƽ�ⳣ��K����K��___(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g)
CO(g)��H2O(g)��ƽ�ⳣ��K����K��___(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g)��___��Ӧ(����ȡ����ȡ�)��
CO(g)��H2O(g)��___��Ӧ(����ȡ����ȡ�)��
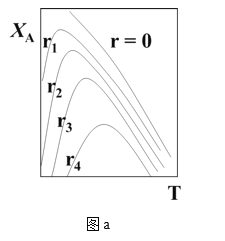
��2��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g) ��H��0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)��CO(g) ��H��0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
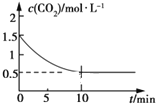
�ٸ������·�Ӧ��ƽ�ⳣ��Ϊ___��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ___mol��L��1��
�����д�ʩ����ʹƽ��ʱ![]() �������___(�����)��
�������___(�����)��
A�������¶� B������ѹǿ
C���ٳ���һ������CO2 D���ټ���һ��������