��Ŀ����
����Ŀ��W��X��Y��ZΪԭ��������������Ķ���������Ԫ�أ�����YԪ����ͬ���������Ӱ뾶��С���ס��ҷֱ���Ԫ��Y��Z�ĵ��ʣ����� ����������W��X��Y��ZԪ����ɵĶ�Ԫ����������¶�ΪҺ̬����Ϊ�������壬������0.01 ![]() ����Һ��pH����2����������ת����ϵ��ͼ��ʾ������˵����ȷ����( )
����Һ��pH����2����������ת����ϵ��ͼ��ʾ������˵����ȷ����( )
A.ԭ�Ӱ뾶��Z>Y>X>W
B.W��X�γɵĻ�����ȿ��ܺ��м��Լ�Ҳ���ܺ��зǼ��Լ�
C.W��X��Y��Z������ͬ����һ�����ӻ�������
D.�Ƚ�X��Z�ǽ�����ǿ��ʱ���ɱȽ�������������Ӧ��ˮ���������
���𰸡�B
��������
W��X��Y��ZΪԭ��������������Ķ���������Ԫ�أ���������������W��X��Y��ZԪ����ɵĶ�Ԫ����������¶�ΪҺ̬����ΪH2O��WΪ��Ԫ�ء��ס��ҷֱ���Ԫ��Y��Z�ĵ��ʣ�����YԪ����ͬ���������Ӱ뾶��С�������һ������ɱ�������ˮ��Ӧ�����죬��Ϊ�������壬������0.01mol��L-1����Һ��pH����2������ҺΪ���ᣬӦ��Al2S3��ˮ��Ӧ����H2S��Al(OH)3���ʱ�ΪAl2S3����ΪH2S����ΪAl(OH)3�����ԭ��������֪XΪ��Ԫ�ء�YΪAl��ZΪ��Ԫ�أ���ΪO2����Ϊ���ʡ�
A��ͬ���ڴ�����ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶��������Ԫ����Hԭ�Ӱ뾶��С��ԭ�Ӱ뾶��Y��Al����Z��S����X��O����W��H������A����
B��W�͢��γɵĻ�������H2O��H2O2��H2O2�мȿ��ܺ��м��Լ�Ҳ���ܺ��зǼ��Լ�����B��ȷ��
C��H��O��Al��S����ͬ����һ�����ӻ������У���KAl(SO4)2��12H2O����C����
D����Ԫ��û������������D����
��ѡB��

����Ŀ����1��ʵ����16 g�״�[CH3OH(l)]�������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�363.25 kJ����������д���״�ȼ���ȵ��Ȼ�ѧ����ʽ��___��
��2���ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡���֪��Ӧ��N2(g)��3H2(g)![]() 2NH3(g) ��H��akJ��mol��1���йؼ����������£�
2NH3(g) ��H��akJ��mol��1���йؼ����������£�
��ѧ�� | H��H | N��H | N��N |
����(kJ��mol��1) | 436 | 391 | 945 |
�Ը��ݱ������м������ݼ���a=____��
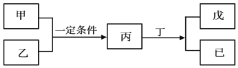
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ�ķ�Ӧ�Ƚ������㡣
��֪��C(s��ʯī)��O2(g)=CO2(g) ��H1����393.5kJ��mol��1
2H2(g)��O2(g)=2H2O(l) ��H2����571.6kJ��mol��1
2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l) ��H3����2599kJ��mol��1
���ݸ�˹���ɣ�����298 Kʱ��C(s��ʯī)��H2(g)����1 mol C2H2(g)��Ӧ�ķ�Ӧ��Ϊ����H��___��