��Ŀ����
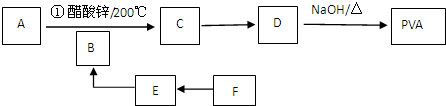
16����A��B��G�����л��������һ����������NaOH��Һ��Ӧ���������ﻹ�ܷ�����ͼ��ʾ�ı仯�����ֲ�����ȥ��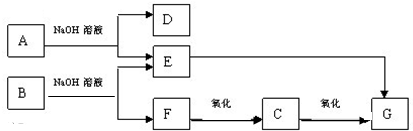
��֪��
��E���ʯ�ҹ��ȣ����Ƶ��ܶ�Ϊ0.717 g/L����̬������״̬�²ⶨ���ܶȣ���
��C�����Ƶ�Cu��OH��2����Һ��������ש��ɫ������
��D��������ˮ��ͨ�������Ϊ��ɫ���д̼�����ζ�����壬��ˮ��Һ��ε���AgNO3��Һ���������ǣ��ټ�������D��Һ���ֱ�Ϊ������Һ�����ó�����Һ���Թ�����C��Ϲ��ȣ�������������
��G��ʹ���Ƶ�Cu��OH��2����Һ����壮
��ش�
��1��д�����и����ʵĽṹ��ʽ��ACH3COONH4��BCH3COOCH2CH3��CCH3CHO��
��2��д�����и���Ӧ�Ļ�ѧ����ʽ��
��A��E��CH3COONH4+NaOH$\stackrel{��}{��}$CH3COONa+NH3��+H2O��
��C�����Ƶ�Cu��OH��2��Ӧ��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O��+2H2O��
��3��д��Fͬ���칹��Ľṹ��ʽ��CH3OCH3��
���� ��״̬���ܶ�Ϊ0.717 g/L����̬������Ħ������Ϊ0.717g/L��22.4L/mol��16g/mol��ΪCH4���Ҵ������ʯ�ҹ��ȷ������ȷ�Ӧ���ɼ��飬����EΪCH3COONa��F��������C��C��������G��E�ܹ�����G��G��ʹ���Ƶ�Cu��OH��2����Һ����壬˵��GΪCH3COOH��FΪCH3CH2OH��CΪCH3CHO����BΪCH3COOCH2CH3��D��������ˮ��ͨ�������Ϊ��ɫ���д̼�����ζ�����壬��ˮ��Һ��ε���AgNO3��Һ���������ǣ��ټ�������D��Һ���ֱ�Ϊ������Һ�����ó�����Һ���Թ�����C��Ϲ��ȣ�������������˵��DΪNH3��AΪCH3COONH4���ݴ˷�����
��� �⣺��״̬���ܶ�Ϊ0.717 g/L����̬������Ħ������Ϊ0.717g/L��22.4L/mol��16g/mol��ΪCH4���Ҵ������ʯ�ҹ��ȷ������ȷ�Ӧ���ɼ��飬����EΪCH3COONa��F��������C��C��������G��E�ܹ�����G��G��ʹ���Ƶ�Cu��OH��2����Һ����壬˵��˵��GΪCH3COOH��FΪCH3CH2OH��CΪCH3CHO����BΪCH3COOCH2CH3��D��������ˮ��ͨ�������Ϊ��ɫ���д̼�����ζ�����壬��ˮ��Һ��ε���AgNO3��Һ���������ǣ��ټ�������D��Һ���ֱ�Ϊ������Һ�����ó�����Һ���Թ�����C��Ϲ��ȣ�������������˵��DΪNH3��AΪCH3COONH4��
��1��AΪCH3COONH4��BΪCH3COOCH2CH3��CΪCH3CHO���ʴ�Ϊ��CH3COONH4��CH3COOCH2CH3��CH3CHO��
��2��AΪCH3COONH4��A��NaOH��Һ��Ӧ����������백������ѧ����ʽΪCH3COONH4+NaOH$\stackrel{��}{��}$CH3COONa+NH3��+H2O��CΪCH3CHO��C�����Ƶ�Cu��OH��2��Ӧ�Ļ�ѧ����ʽCH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O��+2H2O���ʴ�Ϊ��CH3COONH4+NaOH$\stackrel{��}{��}$CH3COONa+NH3��+H2O��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O��+2H2O��
��3��FΪCH3CH2OH����ͬ���칹��ֻ�м���CH3OCH3���ʴ�Ϊ��CH3OCH3��
���� ����ͨ���л���ͼ�ƶϿ���������ˮ�⡢�����������Լ�������Ӧ�ȵ�֪ʶ�㣬ע��ݹ����ŵ������ƶϣ���Ŀ�ѶȲ���

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�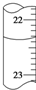 ����˵����ȷ���ǣ�������
����˵����ȷ���ǣ���������ʵ�������þƾ��Ƽ��ȵ���������Բ����ƿ���ձ�
��������100mlʯ�Ϳɽ�������250ml������ƿ��
�����Ƶ���ˮ��������ɫ���ƿ�У�������������
�ܲ�����Һ��CuSO4��ʯ��ˮ��һ��������ϣ�ʢ��������������
�����ζ����е�Һ����ͼ��ʾ���������ӦΪ23.65mL��
| A�� | �٢� | B�� | �٢ۢ� | C�� | �ۢܢ� | D�� | �ڢۢ� |
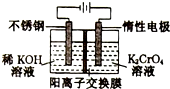 �Ը���أ�������ʼ����0.4molK2CrO4��Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���ص�ʵ��װ��ʾ��ͼ��ͼ������˵����ȷ���ǣ�������
�Ը���أ�������ʼ����0.4molK2CrO4��Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���ص�ʵ��װ��ʾ��ͼ��ͼ������˵����ȷ���ǣ�������| A�� | �������ң�ͨ�����Һ���ɳ�ɫ��Ϊ��ɫ | |
| B�� | ��·����0.2mol����ͨ��ʱ��������������Һ���ٵ�������Ϊ1.4g | |
| C�� | �����������K��Cr�����ʵ���֮��Ϊ3��2����˹��̵�·�й�ת�Ƶ�����Ϊ0.1NA | |
| D�� | ���ⶨ����Һ��K��Cr�����ʵ���֮��Ϊd�����ʱ����ص�ת����Ϊ2-d |

| A�� | ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����X������NaAlO2 | |
| B�� | ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����X������NH3 | |
| C�� | ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ�ǽ�������ʱ����Z������CO2 | |
| D�� | ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ��������ʱ����Z������FeCl3 |
| A�� | �����ʵ�����OH-���ǻ���-OH��������������� | |
| B�� | ���³�ѹ�£�44 g CO2���庬����ԭ�ӵĸ���Ϊ2 NA | |
| C�� | 1 L 0.5 mol•L-1NaHCO3��Һ�к���HCO3-�ĸ���Ϊ0.5 NA | |
| D�� | 11.2 g������ϡ���ᷴӦת�Ƶ�����һ��Ϊ0.6 NA |
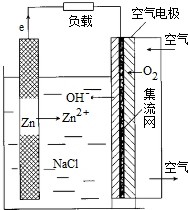
| A�� | �ŵ�ʱ�����������ӦʽΪ��O2+H++4e-=2H2O | |
| B�� | �������������ֱ����������������������ӳ����ʹ������ | |
| C�� | �ŵ�ʱ��ÿͨ��2.24L��������״�����������ϸ�����Ҫ����13gZn | |
| D�� | ��������ֻ�������װ�õ�п�ۻ���п�弴�� |

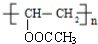 +n NaOH��
+n NaOH��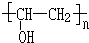 +n CH3COONa��
+n CH3COONa��