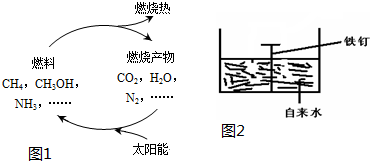
��Ŀ����
9����1������Se�������������Ԫ�أ���֪�����ڱ��У�������ͬ���壬���ͬ���ڣ��Իش��������⣺����λ�����ڱ���4���ڣ�VIA�壻
���������������Ļ�ѧʽΪSeO3����Ӧˮ����Ļ�ѧʽΪH2SeO4��
��2�������ijһ���ҹ����ֳ��п��������ܱ����Ķ����ش��������⣮
| ���� | ��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ |
| ���� | 92 | TSP | �� |
| �Ϻ� | 74 | �� | |
| ���� | 76 | TSP | �� |
| ���� | 98 | �� |
�ڶ��������dz����Ĵ�����Ⱦ��֮һ���ҹ��涨�����ж������������ó���0.02mg/L��Ϊ���ٶ��������ŷ��������д�ʩ�У��ɲ�ȡ����AB��
A������Ȼ������ú̿������ȼ��
B������ȼ��������
C�������ữ�������м�ʯ��
D��ֲ�����֣�
���� ��1�������ǵ�VIA��Ԫ�أ�
�ڸ���������������������ϼۣ�Ȼ����д����������Ӧ��ˮ���ﻯѧʽ��
��2�����������Ⱦָ����ߣ�
��A������ú��ȼ�Ͽ��Լ��ٶ�������������IJ�����
B��ȼ��������Լ��ٶ�������IJ�����
C�������ữ�������м�ʯ��ֻ�ܹ��к��������ʣ�
D��ֲ�����ֲ��ܼ��ٶ���������ŷţ�
��� �⣺��1�������ǵ�VIA��Ԫ�أ���λ�����ڱ���4���ڣ�VIA�壬�ʴ�Ϊ��4��VIA��
������ϼ�=����������=6�����������Ļ�ѧʽΪSeO3����Ӧˮ����Ļ�ѧʽΪH2SeO4���ʴ�Ϊ��SeO3��H2SeO4��
��2�����������Ⱦָ����ߣ������׳������꣬�ʴ�Ϊ�����죻
��A������ú��ȼ�Ͽ��Լ��ٶ�������������IJ������Ӷ���ֹ�������꣬��A��ȷ��
B��ȼ��������Լ��ٶ�������IJ������Ӷ���ֹ�������꣬��B��ȷ��
C�������ữ�������м�ʯ��ֻ�ܹ��к��������ʣ����ܷ�ֹ����ij��֣���C����
D��ֲ�����������ն�����̼�����ܼ��ٶ���������ŷţ���D����
�ʴ�Ϊ��AB��
���� ���⿼��ѧ��Ԫ�����ڱ��Ľṹ�Լ�������Ⱦ����ȷ��������Դ�������Ⱦ��������ʩ����������������ѧ�����Ӧ����ѧ֪ʶ��������

| A�� | ���ڱ������Ԫ�����ڵ�����������ԭ�Ӻ�������� | |
| B�� | ���ڱ��Ԫ�����ڵ�������������ԭ�Ӻ������������� | |
| C�� | Ԫ�����ڱ���7�����壬7�����壬1��0�壬1�����壬��16���� | |
| D�� | X2+�ĺ��������ĿΪ18����X�ڵ������ڵڢ�A�� |
| A�� | �����£���ˮ���Ȼ�淋�pH=7�Ļ����Һ�У�c��Cl-��=c��NH4+�� | |
| B�� | pH=1��һԪ���pH=13��һԪ��������ϣ�c��OH-��=c��H+�� | |
| C�� | 0.1 mol•L-1�ģ�NH4��2SO4��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� | |
| D�� | 0.1 mol•L-1��NaHCO3��Һ�У�c��HCO3-����c��CO32-����c��H2CO3�� |
| ��һ�� | He | -268.8 | ��a�� | -249.5 | Ar | -185.8 | Kr | -151.7 |
| �ڶ��� | F2 | -187.0 | Cl2 | -33.6 | ��b�� | 58.7 | I2 | 184.0 |
| ������ | ��c�� | 19.4 | HCl | -84.0 | HBr | -67.0 | HI | -35.3 |
| ������ | H2O | 100.0 | H2S | -60.2 | ��d�� | -42.0 | H2Te | -1.8 |
| A�� | abc�Ļ�ѧʽ�ֱ�ΪNe2��Br2��HF | |
| B�� | ���������������Ƚϣ���������ȶ�˳��Ϊ��HBr��d | |
| C�� | ��������������ˮ����Һ������c��ǿ | |
| D�� | ������������H2O�ķе���ߣ�����ΪH2O�����ڴ������ |
| A�� | ���������V����V����������V������������V�������� | |
| B�� | ���������V����V���������仯���ұ仯�ı�����ͬ | |
| C�� | ����ѹǿʱ��V����V����������V������������V�������� | |
| D�� | �����¶�ʱ��V����V������С����V����С����С��V����С���� |

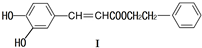 ��ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
��ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
 ����Ӧ���ͣ�ˮ�⣨ȡ������Ӧ
����Ӧ���ͣ�ˮ�⣨ȡ������Ӧ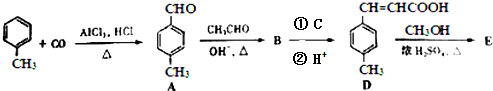
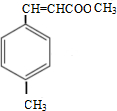 ��
��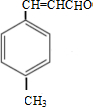 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$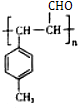 ��
��