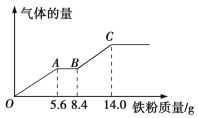
��Ŀ����
����Ŀ����1 L FeCl3��CuCl2�Ļ����Һ������Cu2+Ũ��Ϊ0.05 mol��L��1���м���һ������Fe�ۣ���ַ�Ӧ����ˣ�ʣ���������Ϊ6.0 g������Һ�м���������AgNO3��Һ������57.4 g����������˵����ȷ����
A.ʣ�����ΪCuB.����Fe�۵�����Ϊ8.4 g
C.ԭ��Һ��Fe3+Ũ��Ϊ0.3 mol��L��1D.��Ӧ����Һ��Fe2+Ũ��Ϊ0.2 mol��L��1
���𰸡�BD
��������
57.4 g�Ȼ������������ʵ���Ϊ![]() =0.4mol������ԭ�Ӹ����غ��֪1 L FeCl3��CuCl2�Ļ����Һ�������ӵ����ʵ���Ϊ0.4mol���ɵ���غ�ɵ�n��Cu2+����2+ n��Fe3+����3= n��Cl�����������Һ��n��Cu2+��=0.05 mol����n��Fe3+��=
=0.4mol������ԭ�Ӹ����غ��֪1 L FeCl3��CuCl2�Ļ����Һ�������ӵ����ʵ���Ϊ0.4mol���ɵ���غ�ɵ�n��Cu2+����2+ n��Fe3+����3= n��Cl�����������Һ��n��Cu2+��=0.05 mol����n��Fe3+��=![]() =0.1 mol��
=0.1 mol��
A.�����Һ��ͭ���ӵ����ʵ���Ϊ0.05 mol����ͭԭ�Ӹ����غ��֪����ͭ��ȫ��Ӧ����ͭ������Ϊ0.05 mol��64g/mol=3.2g��6.0 g����ʣ�����ΪCu������Fe����A����
B.�������֪�������Һ�������Ӻ�ͭ���Ӿ���ȫ��Ӧ���ɵ�ʧ������Ŀ�غ�ɵ÷�Ӧ���������ʵ���Ϊ![]() =
=![]() =0.1mol�������Fe�۵�����Ϊ0.1mol��56g/mol+��6.0 g��3.2g��= 8.4 g����B��ȷ��
=0.1mol�������Fe�۵�����Ϊ0.1mol��56g/mol+��6.0 g��3.2g��= 8.4 g����B��ȷ��
C. �����Һ�������ӵ����ʵ���Ϊ0.1mol����1 L FeCl3��CuCl2�Ļ����Һ�������ӵ�Ũ��Ϊ0.1mol/L����C����
D.����ԭ�Ӹ����غ��֪����Ӧ����Һ��n��Fe2+��= n��Fe3+��+ ��Ӧn��Fe��=0.1 mol+0.1 mol=0.2mol����Ӧ����Һ���������ӵ�Ũ��Ϊ0.2mol/L����D��ȷ��
��ѡBD��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ����������1mol��ѧ�������γ���̬ԭ������Ҫ��������![]() ��ʾ����ϱ�����Ϣ�ж�����˵������ȷ����( )
��ʾ����ϱ�����Ϣ�ж�����˵������ȷ����( )
���ۼ� | H-H | F-F | H-F | H-Cl | H-I |
E(kJ/mol) | 436 | 157 | 568 | 432 | 298 |
A. 432kJ/mol>E(H-Br)>298kJ/mol B. �������ȶ��Ĺ��ۼ���H-F��
C. H2(g)��2H(g) ��H=+436kJ/mol D. H2(g)+F2(g)=2HF(g) ��H=-25kJ/mol