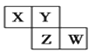
��Ŀ����
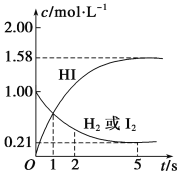
����Ŀ��N2O5��һ����������������һ���¶��¿��Է������·�Ӧ��2N2O5(g) ![]() 4NO2(g)+O2(g) ��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ�����
4NO2(g)+O2(g) ��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ�����
![]()
����˵���в���ȷ����
A.500s��N2O5�ֽ�����Ϊ2.96��10-3mol��L-1��s-1
B.T1�¶��µ�ƽ�ⳣ��ΪK1=125��ƽ��ʱN2O5��ת����Ϊ50%
C.T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1 >T2����K1>K2
D.ƽ��������������䣬���������ѹ����ԭ����![]() ����c(NO2)<5.00mol��L-1
����c(NO2)<5.00mol��L-1
���𰸡�D
��������
A.����ͼ�����ݷ�������500s��N2O5(g)���ĵ�Ũ��=5.00mol/L-3.52mol/L=1.48mol/L������ֽ�����V=![]() =Ϊ2.96��10-3 mol/(Ls)��A��ȷ��
=Ϊ2.96��10-3 mol/(Ls)��A��ȷ��
B.�ɱ������ݿ�֪��T1�¶��£�1000sʱ��Ӧ����ƽ�⣬ƽ��ʱc(N2O5)=2.5mol/L��c(NO2)=5mol/L��c(O2)=1.25mol/L��ƽ�ⳣ��K=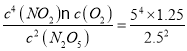 =125��ת����Ϊ
=125��ת����Ϊ![]() ��100%=50%��B��ȷ��
��100%=50%��B��ȷ��
C.�÷�Ӧ������Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�ƶ���ƽ�ⳣ������C��ȷ��
D.T1�¶��£�1000sʱ��Ӧ����ƽ�⣬ƽ��ʱc(NO2)=5mol/L����ƽ��������������䣬�����������ѹ����ԭ����![]() ����ƽ��ŭ�����ƶ�����c(NO2)=10mol/L������ѹƽ�������������С���淴Ӧ�����ƶ�����ƽ���ƶ������ģ��ܵ���˵����Ũ�ȱ�ԭ������������10mol/L>c(NO2)5.00mol��L��D����
����ƽ��ŭ�����ƶ�����c(NO2)=10mol/L������ѹƽ�������������С���淴Ӧ�����ƶ�����ƽ���ƶ������ģ��ܵ���˵����Ũ�ȱ�ԭ������������10mol/L>c(NO2)5.00mol��L��D����
�ʺ���ѡ����D��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�