��Ŀ����
6�������±��ṩ���������г��������Լ���ѡ��������ʵ����Ӧʵ��Ŀ���ǣ�������| ѡ�� | ʵ��Ŀ�� | ���� |
| A | ��ʳ��ˮ�л��NaCl���� | �������������ƾ��� |
| B | ��ϡH2SO4��Na2CO3��Һ�Ƚ�Ԫ��S��C�ķǽ�����ǿ�� | �Թܡ���ͷ�ι� |
| C | ��8.0mol��L-1���������� 250mL1.5mol��L-1������ | 250m����ƿ�����������ձ� |
| D | �ⶨNaOH��Һ�����ʵ���Ũ�� | �ᣨ�ʽ�ζ��ܣ���ͷ�ιܡ���ƿ���ձ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A����ʳ��ˮ�л��NaCl���壬ѡ����������
B������ǿ����ȡ����ķ�Ӧ�ȽϷǽ����ԣ�
C��������Ҫ��ͷ�ιܣ�
D���ⶨNaOH��Һ�����ʵ���Ũ�ȣ������к͵ζ�ʵ�飮
��� �⣺A����ʳ��ˮ�л��NaCl���壬ѡ������������Ҫ�������������ƾ��ƣ���A��ȷ��
B������ǿ����ȡ����ķ�Ӧ�ȽϷǽ����ԣ����Թ������ý�ͷ�ιܵζ����ɣ���B��ȷ��
C��������Һ��ת�Ƶ�����ƿ�ж�����Ҫ��ͷ�ιܣ�ȱ�ٽ�ͷ�ιܣ�������ɣ���C����
D���ⶨNaOH��Һ�����ʵ���Ũ�ȣ������к͵ζ�ʵ�飬��Ҫ�ᣨ�ʽ�ζ��ܣ���ͷ�ιܡ���ƿ���ձ�����D��ȷ��
��ѡC��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰���������ᴿ���ζ�ʵ�顢��Һ���Ƶȣ��������ʵ����ʡ�ʵ�����������Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
�����Ŀ
16�����н���ʵ����ʵ�ķ���ʽ��ȷ���ǣ�������
| A�� | ������Һ������ϴ���۵�ԭ��CO32-+2H2O?H2CO3+2OH- | |
| B�� | ��ǿ����Һ��NaClO��Fe��OH��3��Ӧ�Ʊ�Na2FeO4��3ClO-+2Fe��OH��3+4OH?=2FeO42-+3Cl-+5H2O | |
| C�� | ͭ��Ʒ�����ˮĤ���Խ�ǿʱ�������绯ѧ��ʴ��������ӦΪ2H++2e-=H2�� | |
| D�� | ��AgNO3��Һ�мӹ���NaCl���ټ�Na2S��Һ����ɫ�������ɫ 2Ag++S2-=Ag2S�� |
17��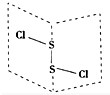 ���Ȼ���S2Cl2���ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��һ�ֳȻ�ɫ��Һ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壮��ѧ����ʽΪ2S2Cl2+2H2O�TSO2��+3S��+4HCl������˵���д�����ǣ�������
���Ȼ���S2Cl2���ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��һ�ֳȻ�ɫ��Һ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壮��ѧ����ʽΪ2S2Cl2+2H2O�TSO2��+3S��+4HCl������˵���д�����ǣ�������
 ���Ȼ���S2Cl2���ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��һ�ֳȻ�ɫ��Һ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壮��ѧ����ʽΪ2S2Cl2+2H2O�TSO2��+3S��+4HCl������˵���д�����ǣ�������
���Ȼ���S2Cl2���ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��һ�ֳȻ�ɫ��Һ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壮��ѧ����ʽΪ2S2Cl2+2H2O�TSO2��+3S��+4HCl������˵���д�����ǣ�������| A�� | S2Cl2�ĵ���ʽΪ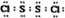 | |
| B�� | ��Ӧ��SO2�ǻ�ԭ���S���������� | |
| C�� | S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ļ��Է��� | |
| D�� | ��Ӧ�У�����1molSO2��ת�Ƶ���Ϊ3mol |
14����NA�ǰ����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | ���³�ѹ�£�6.4g O2��O3�Ļ�������к��еķ�����Ϊ0.4 NA | |
| B�� | ��״���£�2.24 L CCl4�����й��ۼ�����ĿΪ0.4NA | |
| C�� | 25��ʱ��pH=13��Ba��OH��2��Һ�к���������������Ϊ0.1 NA | |
| D�� | �����£�16.8 g Fe������ˮ������ȫ��Ӧʧȥ0.8NA������ |
1�� W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������
 W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������| A�� | ��Ӧ�����Ӱ뾶��W��X | |
| B�� | ��Ӧ��̬�⻯����ȶ��ԣ�Y��Z | |
| C�� | ������XZW�Ⱥ����Ӽ����ֺ����ۼ� | |
| D�� | Z���⻯���X������������Ӧˮ�������Һ������Y�������ﷴӦ |
11������˵������ȷ���ǣ�������
| A�� | ͬ���ڽ���Ԫ�أ�ԭ��ʧ��������Խǿ������ϼ�Խ�� | |
| B�� | ͬ����ĵ��������ӣ��仹ԭ��Խǿ��ˮ��̶�Խ�� | |
| C�� | IA��VIIA��Ԫ�ؼ���γɹ��ۻ����� | |
| D�� | �ڶ�����Ԫ�ش���������ϼ۴�+1��+7 |
18��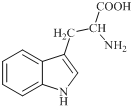 ɫ������ֲ����������������ϳ���Ҫ��ǰ�����ʣ��ձ�����ڸߵ�ֲ���У�������ɫ����Ϊ��ɫ����ɫ�ᾧ������ˮ������������������ƣ���ṹ��ͼ��ʾ�����й���ɫ�����˵������ȷ���ǣ�������
ɫ������ֲ����������������ϳ���Ҫ��ǰ�����ʣ��ձ�����ڸߵ�ֲ���У�������ɫ����Ϊ��ɫ����ɫ�ᾧ������ˮ������������������ƣ���ṹ��ͼ��ʾ�����й���ɫ�����˵������ȷ���ǣ�������
 ɫ������ֲ����������������ϳ���Ҫ��ǰ�����ʣ��ձ�����ڸߵ�ֲ���У�������ɫ����Ϊ��ɫ����ɫ�ᾧ������ˮ������������������ƣ���ṹ��ͼ��ʾ�����й���ɫ�����˵������ȷ���ǣ�������
ɫ������ֲ����������������ϳ���Ҫ��ǰ�����ʣ��ձ�����ڸߵ�ֲ���У�������ɫ����Ϊ��ɫ����ɫ�ᾧ������ˮ������������������ƣ���ṹ��ͼ��ʾ�����й���ɫ�����˵������ȷ���ǣ�������| A�� | ɫ����ķ���ʽΪC11H12N2O2 | |
| B�� | ɫ�����ܷ���ȡ�����ӳɡ��������кͷ�Ӧ | |
| C�� | ɫ��������ˮ��������������Һ����Ϊ�����ᡢ��ܷ�Ӧ������ | |
| D�� | ��ɫ������ʰ��ᣨNH2CH2COOH����ϣ���һ�������������γ����ֶ��� |
15������װ��Ӧ����ʵ���ҴӷϾɵĺ�CuI/SiO2��������ȡ���ʵ�飬���ܴﵽʵ��Ŀ���ǣ�������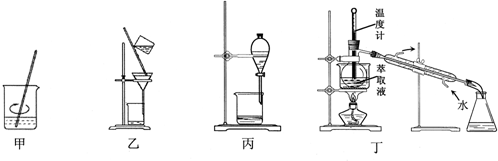

| A�� | ��װ�ü�ϡH2SO4��H2O2��������CCl4��ȡ | |
| B�� | ��װ���ҹ��˷������ȡҺ | |
| C�� | ��װ�ñ�����õ�������Ȼ�̼��Һ | |
| D�� | ��װ�ö�������ȡҺ�е��ʵ�����Ȼ�̼ |
 ��
��