��Ŀ����
1�� W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������| A�� | ��Ӧ�����Ӱ뾶��W��X | |
| B�� | ��Ӧ��̬�⻯����ȶ��ԣ�Y��Z | |
| C�� | ������XZW�Ⱥ����Ӽ����ֺ����ۼ� | |
| D�� | Z���⻯���X������������Ӧˮ�������Һ������Y�������ﷴӦ |
���� W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ�W��һ�ֺ��ص�������Ϊ18��������Ϊ10����֪W��������Ϊ8����W����Ԫ�أ�X��Neԭ�ӵĺ�����������1����ԭ�Ӱ뾶��W��֪XΪ11��Ԫ�أ���XΪNaԪ�أ�Y��ԭ�Ӱ뾶����X��W֮�䣬Y�ĵ�����һ�ֳ����İ뵼����ϣ�����Y��SiԪ�أ�Z�ķǽ�������ͬ����Ԫ������ǿ��ԭ����������Si����ZΪClԪ�أ��ݴ˽��
��� �⣺W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ�W��һ�ֺ��ص�������Ϊ18��������Ϊ10����֪W��������Ϊ8����W����Ԫ�أ�X��Neԭ�ӵĺ�����������1����ԭ�Ӱ뾶��W��֪XΪ11��Ԫ�أ���XΪNaԪ�أ�Y��ԭ�Ӱ뾶����X��W֮�䣬Y�ĵ�����һ�ֳ����İ뵼����ϣ�����Y��SiԪ�أ�Z�ķǽ�������ͬ����Ԫ������ǿ��ԭ����������Si����ZΪClԪ�أ�
A��O2-��Na+���ӵ��Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��O2-��Na+����A��ȷ��
B���ǽ�����Si��Cl���ǽ�����Խǿ���⻯��Խ�ȶ�����B��ȷ��
C��������NaClO�Ⱥ����Ӽ����ֺ����ۼ�����C��ȷ��
D��Y��������Ϊ�������裬Z���⻯��ΪHCl��X���������Ӧ��ˮ����ΪNaOH������������������������Һ��Ӧ�����������ᷴӦ����D����
��ѡD��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ؼ��Ǹ���ԭ�Ӱ뾶��ԭ������ȷ��Ԫ�أ�ע��Ի���֪ʶ���������գ�

| A�� | H2SO4��ϡ��$��_{��}^{Cu}$SO2$\stackrel{������ˮ}{��}$NH4HSO3$\stackrel{������ˮ}{��}$��NH4��2SO3 | |
| B�� | NH3$��_{������}^{O_{2}}$NO$\stackrel{O_{2}}{��}$NO2$\stackrel{H_{2}O}{��}$HNO3 | |
| C�� | Fe$��_{��}^{����Cl_{2}}$FeCl2$\stackrel{NaOH��Һ}{��}$Fe��OH��2$\stackrel{�����з���}{��}$Fe��OH��3 | |
| D�� | Al$\stackrel{NaOH��Һ}{��}$NaAlO2$\stackrel{��������}{��}$AlCl3��Һ$\stackrel{��}{��}$��ˮAlCl3 |
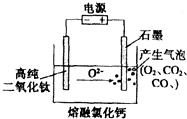 ��ͼΪEFC���ŷ��ù���������ѣ�TiO2�����������ѵ�װ��ʾ��ͼ����ԭ�����ڽϵ͵�������λ�£�TiO2���������е���������������Σ��������ֻʣ�´��ѣ�����˵������ȷ���ǣ�������
��ͼΪEFC���ŷ��ù���������ѣ�TiO2�����������ѵ�װ��ʾ��ͼ����ԭ�����ڽϵ͵�������λ�£�TiO2���������е���������������Σ��������ֻʣ�´��ѣ�����˵������ȷ���ǣ�������| A�� | �����ĵ缫��ӦʽΪ��2Cl--2e-=Cl2�� | |
| B�� | ͨ���O2-��Cl-���������ƶ� | |
| C�� | �����ĵ缫��ӦʽΪTiO2+4e-=Ti+2O2- | |
| D�� | ʯī�缫�������������仯 |
| A�� | �ع��ͺͿ����Ͷ������������� | |
| B�� | ��ʳƷ���з���ʢ�й轺�����۵���С�����ɷ�ֹʳ���ܳ����������� | |
| C�� | Ϊ�ⶨ�����������Ƶĵ����ԣ����������ƹ������ʯӢ�����м����ۻ� | |
| D�� | �����ʡ��������ۡ���֬�����ɸ߷�����ɵ����� |
| A�� | ����ͭ�����ڵ����壬����������ģ������ʹ�õ�ʱ��Զ����ͭ��������ģ������ʹ�õ�ʱ�� | |
| B�� | ʹ��úҺ���IJ�Ʒ��ȼ����ֱ��ȼ��ú�Ƚϣ��Ա�������������Ŀǰ��úҺ����Ψһ;���ǽ�ú�ڸ��ºʹ�����������������Ӧ | |
| C�� | ʯ�ͷ�������֮һ��ʯ������ʯ�������д����ļ��� | |
| D�� | ���մɡ��ۺ����м����̬�������ӣ����ԸĽ����ϵ��ͳ��ǿ�Ⱥ��Ͷ���ǿ�� |
| ѡ�� | ʵ��Ŀ�� | ���� |
| A | ��ʳ��ˮ�л��NaCl���� | �������������ƾ��� |
| B | ��ϡH2SO4��Na2CO3��Һ�Ƚ�Ԫ��S��C�ķǽ�����ǿ�� | �Թܡ���ͷ�ι� |
| C | ��8.0mol��L-1���������� 250mL1.5mol��L-1������ | 250m����ƿ�����������ձ� |
| D | �ⶨNaOH��Һ�����ʵ���Ũ�� | �ᣨ�ʽ�ζ��ܣ���ͷ�ιܡ���ƿ���ձ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | pH=1����ɫ��Һ�У�K+��NH4+��SO42-��MnO4- | |
| B�� | ˮ�������c��OH-��=10-12 mo1•L-1����Һ�У�Fe2+��Ba2+��NO3-��ClO- | |
| C�� | ������A13+����Һ�У�Na+��SO42-��NO3-��[Al��OH��4]- | |
| D�� | CO2�ı�����Һ�У�K+��Ca2+��Cl-��NO3- |
| A�� | 4.6������ˮ��Ӧʱʧȥ�ĵ�����ĿΪ0.2NA | |
| B�� | 1 L0.5mol•L-1 Na2SO4��Һ�������е�Na+��������ΪNA | |
| C�� | �ڱ�״���£�22.4LNH3�����İ�������ĿΪNA | |
| D�� | ���³�ѹ��2����������ԭ������ΪNA |

��֪����2NO+Na2O2=2NaNO2��
��3NaNO2+3HCl=3NaCl+HNO3+2NO��+H2O��
�����������£�NO��NO2������MnO4-��Ӧ����NO3-��Mn2+��Na2O2��ʹ���Ը��������Һ��ɫ��
��1������װ��Aǰ����ͨһ��ʱ��N2��Ŀ�����ų�װ���еĿ�����
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��ʵ�������Bƿ�е���Һ������Ũ������ȴ�ᾧ����������ƣ������˿ɻ��CuSO4•5H2O��
��3������C������Ϊ����ܣ�����ʢ�ŵ�ҩƷΪ��ʯ�ң������ƣ���
��4����ַ�Ӧ����װ��D�в���ķ����ǣ�ȡ�������������Թ��У�����ϡ������Һ�������ݲ��������Թܿ��Ϸ����ֺ���ɫ���壬�������NaNO2��ע���Լ�������
��5��Ϊ�ⶨ�������Ƶĺ�������ȡ4.000g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol•L-1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
b����ƿϴ����δ����
c���ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݣ��������ù������������Ƶ���������86.25%��
��6����ƺ���ʵ��Ƚ�0.1mol•L-1NaNO2��Һ��NO2-��ˮ��̶Ⱥ�0.1mol•L-1HNO2��Һ��HNO2�ĵ���̶���Դ�С������Ҫ˵��ʵ�鲽�衢����ͽ��ۣ�������ҩƷ��ѡ��
25��C��