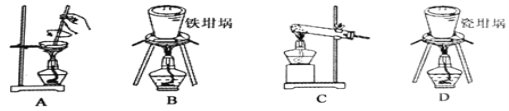
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΓΑ…ζΜνΈό¥Π≤ΜΜ·―ßΓ±Θ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
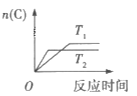
Θ®1Θ©”ΟΑΉ¥ΉΫΰ≈ίΩΣΥ°ΚχΘ§ΒΎΕΰΧλΖΔœ÷ΚχΡΎΥ°ΙΗœϊ ßΘ§ΩΣΥ°Κχ”÷ΙβΫύ»γ–¬ΝΥΘ®Υ°ΙΗΒΡ÷ς“Σ≥…Ζ÷ «ΧΦΥαΗΤΒ»Θ©ΓΘΗΟάκΉ”Ζ¥”ΠΖΫ≥Χ ΫΈΣ____________ΓΘ
Θ®2Θ© ≥―Έ≤Μ…ς»ς¬δ‘ΎΧλ»ΜΤχΒΡΜπ―φ…œΘ§Ιέ≤λΒΫΒΡœ÷œσ «_____Θ§ΗΟ±δΜ·≥ΤΈΣ_____Ζ¥”ΠΓΘ
Θ®3Θ©’¥”–Υ°ΒΡΧζ÷ΤΤςΟσ‘ΎΗΏΈ¬Μπ―φ…œΜαΖΔΚΎΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «____________ΓΘ
Θ®4Θ©ΟφΑϋ‘Ύ÷ΤΉς ±ΜαΦ”»κ–ΓΥ’¥ρΘ§άϊ”ΟΤδΦ”»»≤ζ…ζΤχΧεΒΡ–‘÷ Θ§Ω…ΫΪ–ΓΥ’¥ρΉςΈΣ≈ρΥ…ΦΝΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «________________ΓΘ
Θ®5Θ©ΙβΒΦœΥΈ§ΓΔ…≥Ή”ΓΔ ·”ΔΚΆ¬ξηßΒΡ÷ς“Σ≥…Ζ÷ «__________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®6Θ©…Ά–Ρ‘ΟΡΩΒΡΒώΜ®≤ΘΝß «”Ο__________Θ®ΧνΟϊ≥ΤΘ©Ε‘≤ΘΝßΫχ––ΩΧ ¥Εχ÷Τ≥…ΒΡΓΘ
Θ®7Θ© Ι”ΟΓΑ84Γ±œϊΕΨ“ΚΘ®Κ§NaClOΘ© ±Θ§Α¥“ΜΕ®±»άΐΫΪΥϋ”κΥ°ΜλΚœΘ§≤Δ‘ΎΩ’Τχ÷–Ϋΰ≈ί“ΜΕΈ ±ΦδΘ§ ΙNaClO”κH2OΦΑΩ’Τχ÷–ΒΡCO2≥δΖ÷Ζ¥”ΠΘ§“‘¥οΒΫ…±ΨζœϊΕΨΒΡ–ßΙϊΗϋΚΟΒΡΡΩΒΡΓΘΫΪΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ≤Ι≥δΆξ’ϊΘΚ![]() ____+____ΓΘ
____+____ΓΘ
ΓΨ¥πΑΗΓΩ![]() Μπ―φ±δ≥…ΜΤ…Ϊ ―φ…Ϊ
Μπ―φ±δ≥…ΜΤ…Ϊ ―φ…Ϊ ![]()
![]()
![]() Μρ(3Fe+2O2
Μρ(3Fe+2O2![]() Fe3O4)
Fe3O4) ![]()
![]()
![]() SiO2 «βΖζΥα HClO HCO3ΓΣ
SiO2 «βΖζΥα HClO HCO3ΓΣ
ΓΨΫβΈωΓΩ
ΘΚ(1)ΖΔ…ζΖ¥”ΠΈΣCaCO3+2CH3COOH®T(CH3COO)2Ca+H2O+CO2ΓϋΘ§ΤδάκΉ”Ζ¥”ΠΈΣCaCO3+2CH3COOH®TCa2++2CH3COOΘ≠+H2O+CO2ΓϋΘ§
Ι ¥πΑΗΈΣΘΚCaCO3+2CH3COOH®TCa2++2CH3COOΘ≠+H2O+CO2ΓϋΘΜ
(2) ≥―Έ≤Μ…ς»ς¬δ‘ΎΧλ»ΜΤχΒΡΜπ―φ…œΘ§Ιέ≤λΒΫΒΡœ÷œσ «Μπ―φ±δ≥…ΜΤ…ΪΘ§ΗΟ±δΜ·≥ΤΈΣ―φ…ΪΖ¥”ΠΘ§
Ι ¥πΑΗΈΣΘΚΜπ―φ±δ≥…ΜΤ…ΪΘΜ―φ…ΪΘΜ
(3)’¥”–Υ°ΒΡΧζ÷ΤΤςΟσ‘ΎΗΏΈ¬Μπ―φ…œΜαΖΔΚΎΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «3Fe+4H2O(g)![]() Fe3O4+4H2Θ§Ι ¥πΑΗΈΣΘΚ3Fe+4H2O(g)
Fe3O4+4H2Θ§Ι ¥πΑΗΈΣΘΚ3Fe+4H2O(g)![]() Fe3O4+4H2ΘΜ
Fe3O4+4H2ΘΜ
(4)ΟφΑϋ‘Ύ÷ΤΉς ±ΜαΦ”»κ–ΓΥ’¥ρΘ§άϊ”ΟΤδΦ”»»≤ζ…ζΤχΧεΒΡ–‘÷ Θ§Ω…ΫΪ–ΓΥ’¥ρΉςΈΣ≈ρΥ…ΦΝΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «2NaHCO3![]() Na2CO3+H2O+CO2ΓϋΘ§Ι ¥πΑΗΈΣΘΚ2NaHCO3
Na2CO3+H2O+CO2ΓϋΘ§Ι ¥πΑΗΈΣΘΚ2NaHCO3![]() Na2CO3+H2O+CO2ΓϋΘΜ
Na2CO3+H2O+CO2ΓϋΘΜ
(5)ΙβΒΦœΥΈ§ΓΔ…≥Ή”ΓΔ ·”ΔΚΆ¬ξηßΒΡ÷ς“Σ≥…Ζ÷ «SiO2Θ§Ι ¥πΑΗΈΣΘΚSiO2ΘΜ
(6)…Ά–Ρ‘ΟΡΩΒΡΒώΜ®≤ΘΝß «”Ο«βΖζΥαΕ‘≤ΘΝßΫχ––ΩΧ ¥Εχ÷Τ≥…ΒΡΘ§Ι ¥πΑΗΈΣΘΚ«βΖζΥαΘΜ
(7) Ι”ΟΓΑ84Γ±œϊΕΨ“Κ(Κ§NaClO) ±Θ§Α¥“ΜΕ®±»άΐΫΪΥϋ”κΥ°ΜλΚœΘ§≤Δ‘ΎΩ’Τχ÷–Ϋΰ≈ί“ΜΕΈ ±ΦδΘ§ ΙNaClO”κH2OΦΑΩ’Τχ÷–ΒΡCO2≥δΖ÷Ζ¥”ΠΘ§“‘¥οΒΫ…±ΨζœϊΕΨΒΡ–ßΙϊΗϋΚΟΒΡΡΩΒΡΘ§”…ΒγΚ… ΊΚψΦΑ‘≠Ή” ΊΚψΩ…÷ΣΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣClOΘ≠+CO2+H2O=HClO+HCO3Θ≠Θ§Ι ¥πΑΗΈΣΘΚHClOΘΜHCO3Θ≠ΓΘ

 ΤΎΡ©ΚΟ≥…Φ®œΒΝ–¥πΑΗ
ΤΎΡ©ΚΟ≥…Φ®œΒΝ–¥πΑΗ 99Φ”1Νλœ»ΤΎΡ©ΧΊ―ΒΨμœΒΝ–¥πΑΗ
99Φ”1Νλœ»ΤΎΡ©ΧΊ―ΒΨμœΒΝ–¥πΑΗ ΑΌ«ΩΟϊ–ΘΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
ΑΌ«ΩΟϊ–ΘΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ ΚΟ≥…Φ®1Φ”1ΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
ΚΟ≥…Φ®1Φ”1ΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ Ϋ𹥑ΣΦ®”≈ΚΟΨμœΒΝ–¥πΑΗ
Ϋ𹥑ΣΦ®”≈ΚΟΨμœΒΝ–¥πΑΗ