��Ŀ����
1���ߴ��������õ���չ�ԡ������ԡ������ܡ��������ܺ���ʴ���ܵ��������ܣ����������ߴ������о�Խ��Խ�ܵ����ǹ�ע��ij�о�С�����õ�ⷨ�Ʊ��{�������о��������£�����1�����Һ�����ƣ���ʵ��Ҫ��Ҫ���Ƹ�Ũ�ȵ��������������ƺú������ܷⱣ����Ŀ�ģ���ֹ����
��2��ʵ��̽����Һ��pH��Fe+2+��Ũ�ȶ�ʵ���Ӱ�죬��̽����ѵĵ���Ʊ��ߴ�����������ʵ����õ�һ����������õĽṹ��������2��ͼ�Σ���������Ч��Խ�ߣ����Ч��Խ�ã�

��ʵ��̽������ѹ�������Ϊ���ٵ��Һ��ʼpH3��4��Fe2+��Ũ��ΪCg/Lt
A.30��400 B.40��90 C.90��100
��3�����о�С��ԡ����Һ��ʼpH�Բ�Ʒ��Ӱ�족���˽�һ�i̽��pH��3ʱ������
����������ͬʱ�����������ݲ������t�����������ӷ���ʽ���� Fe2++2e-=Fe�⣬����2H++2e-=H2��
��4����pH=5��6ʱ�����õ��ߴ�����Ч�ʼ��ͣ��������·��������������������о�С
��������¼���
����һ���ó���ΪFe��OH��
��������ó���ΪFe��OH��3
���������ó���ΪFe��OH��2��Fe��OH��3
Ϊ����֤���裬ȡ�����·��������Թ��У�Ϊ��ֹ��������������ȡ�����Լ�ʵʵ��ʱҪ��ȡ�ʵ�����������������Ȼ��
| ���� | ���� | ���� |
| ��������ȷ |
���� ��1�����������Ӿ���ǿ�Ļ�ԭ�ԣ����ױ�����������
��2��������������Ч��Խ�ߣ����Ч��Խ�ã����ͼ2�жϽ��
��3������ȥ���д����Ķ��������ӡ������ӣ����߶��ܵõ����ӷ�����ԭ��Ӧ��
��4�����������ӡ����������Ӷ��ܹ�����������Ӧ���������������������������������Ըó�������Ϊ����������������Ϊ��������������Ϊ������
����������������������Ժ�ɫ�����������Ӿ���ǿ�Ļ�ԭ���ܹ�ʹ���Եĸ��������ɫ��
��� �⣺��1�����������Ӿ���ǿ�Ļ�ԭ�ԣ����ױ������������������ƺú������ܷⱣ��
�ʴ�Ϊ����ֹ������
��2������ͼ2��֪����Fe2+��Ũ��Ϊ��90��100��g/Lt����������Ч��Խ�ߣ����Ч��Խ�ã�
�ʴ�Ϊ��C��
��3������ȥ���д����Ķ��������ӡ������ӣ����߶��ܵõ����ӷ�����ԭ��Ӧ�����Է����ĵ缫��ӦʽΪ��Fe2++2e-=Fe��2H++2e-=H2����
�ʴ�Ϊ��2H++2e-=H2����
��4�����������ӡ����������Ӷ��ܹ�����������Ӧ���������������������������������Ըó�������Ϊ����������������Ϊ��������������Ϊ������
����������������������Ժ�ɫ�����������Ӿ���ǿ�Ļ�ԭ���ܹ�ʹ���Եĸ��������ɫ�����Կ�������������ؼ������������Ӵ��ڣ��������Եĸ�����ؼ�����������Ӵ��ڣ�����������������������Ӷ����������Ϊ���������������������Ļ���
�ʴ�Ϊ��Fe��OH��2��Fe��OH��3��
| ���� | ���� | ���� |
| ���Թ��м���ϡ����ʹ�����ܽ⣬�ֳ�2�ݣ�һ�ݵμ�KSCN����һ�ݵμӸ��������Һ | �μ�KSCN�Թܱ�죬�μӸ�����صĺ�ɫѸ����ɫ |
���� ���⿼���˵��ع���ԭ����ʵ�鷽������ƣ���Ϥ�������������������������ʵIJ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 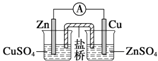 װ�ã�����п-ͭԭ��� | |
| B�� | 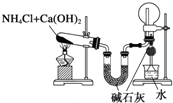 װ�ã�ʵ�����ư������ռ�����İ��� | |
| C�� | 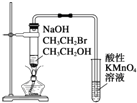 װ�ã���֤�����鷢����ȥ��Ӧ����ϩ�� | |
| D�� | 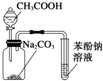 װ�ã���ʵ��������֪����ǿ��˳��ΪCH3COOH��H2CO3��C6H5OH |
| A�� | K+��H+��ClO-��I- | B�� | Fe3+��Mg2+��NO3-��CO32- | ||
| C�� | K+��Ca2+��HCO3-��OH- | D�� | Al3+��NH4+��Cl-��SO42- |
| A�� | ����������ͭ����ȩ��Ӧ�����ӷ���ʽ��CH3CHO+2Cu��OH��2+OH- $��_{����}^{ˮԡ}$ CH3COO-+Cu2O��+3H2O | |
| B�� | ������̼������Һ��Ӧ��C6H5OH+CO32-��C6H5O-+HCO3- | |
| C�� | ����1 mol KAl��SO4��2����Һ�м���Ba��OH��2��Һ�������������ʱ���������ܵ����ʵ���Ϊ2mol | |
| D�� | ������Ũ�����ữ��KMnO4��Һ��H2O2��ϣ���֤��H2O2���л�ԭ�ԣ� 2MnO4-+6H++5H2O2�T2Mn2++5O2��+8H2O |
| A�� | H2OΪֱ���ͷ��� | |
| B�� | ��Ӧ��ÿ����1molSת����2mol���� | |
| C�� | NaHS�к����Ӽ��ͷǼ��Լ� | |
| D�� | ���ʣ�S8��Ϊԭ�Ӿ��� |
| A�� | ����������������ɢϵ�ı��������Ǿ��ж�������� | |
| B�� | ú��������ʯ�ͷ���ˮ��þ���������ȹ����ж�������ѧ�仯 | |
| C�� | ����ľ�ĵ���Ҫ�ɷֶ�����ά�أ���˿����ë������˿����Ҫ�ɷֶ��ǵ����� | |
| D�� | �ױ��ܹ������Ը��������Һ�����ɱ����ᣬ�����鲻��Ӧ��˵�������ܹ�ʹ������� |
| A�� | ���к��ȵIJⶨʵ���У�������������ҺѸ�ٵ���ʢ����������ȼ��У�������������¼��Һ����ʼ�¶ȣ���ַ�Ӧ���ٶ�������¼��Ӧ��ϵ������¶� | |
| B�� | ��װ��2mL 2mol/L AlCl3��Һ���Թ��У���μ���0.01mol/L��ˮ3mL�����������ҳ������ܽ⣬˵�������������������� | |
| C�� | ��Zn-Cuԭ����м���˫��ˮ�������������ҳ���ʱ��ϳ� | |
| D�� | �������ճɻҽ���ˮ���2��3 min�����ˣ���Һ�п��Լ���������H2O2������I? |
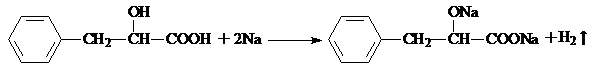
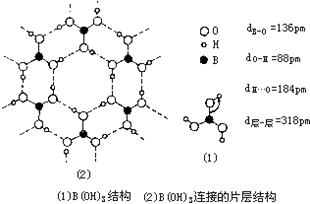 ���仯�������ִ���ҵ���������������ҪӦ�ü�ֵ��
���仯�������ִ���ҵ���������������ҪӦ�ü�ֵ��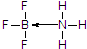 ��
��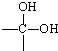 ��
��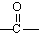 +H2O��A������ʽΪC9H8������ͼ��ת����ϵ��
+H2O��A������ʽΪC9H8������ͼ��ת����ϵ��
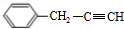 ��ָ����Ӧ�ٵķ�Ӧ���ͣ��ӳɷ�Ӧ��
��ָ����Ӧ�ٵķ�Ӧ���ͣ��ӳɷ�Ӧ�� ��
�� ��
��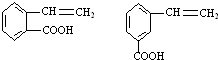 �������������
�������������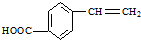 ��
��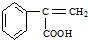 ��
��