��Ŀ����
9��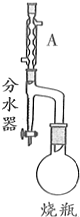 ʵ�����Ʊ�����ϩ������ķ�Ӧװ��ʾ��ͼ���й���Ϣ���£�
ʵ�����Ʊ�����ϩ������ķ�Ӧװ��ʾ��ͼ���й���Ϣ���£�
| ҩƷ | ��Է������� | �۵�/�� | �е�/�� | �ܽ��� | �ܶȣ�g•cm-3�� |
| �״� | 32 | -98 | -64.5 | ��ˮ���ܣ��������л��ܼ� | 0.79 |
| ����ϩ�� | 86 | 15 | 161 | ������ˮ���������л��� | 1.01 |
| ����ϩ����� | 100 | -48 | 100 | ����ˮ���������л��ܼ� | 0.944 |
��100mL��ƿ�����μ��룺15mL����ϩ�ᡢ2����ʯ��10mL��ˮ�״���������Ũ�����ͼʾ����װ�ü�����ƿ�л��Һ��ͨ����ˮ�������ˮ������ƿʢ���ռ�������ˮ����������ˮ���ɣ�ֹͣ���ȣ���ȴ�����Լ�Xϴ����ƿ�еĻ����Һ�����룻ȡ�л�����Һ��ѹ���õ��ϴ����ļ���ϩ���������ش��������⣺
��1��Aװ�õ����������������ܣ�
��2������ʵ����Ũ�����������ϩ��������ʵ����ã���ԭ��Ũ�������ղ���ˮ����ʹ������Ӧ���������ƶ�������Ũ������࣬���ʽ��ͣ�ԭ����Ũ�������ǿ�����ԣ��������л���Ӧ�
��3������ʵ��������ɵĸ�����ṹ��ʽΪCH3OCH3����һ�ּ��ɣ�
��4���Լ�X��ѡ��c��
a������ʳ��ˮ b������������Һ c������̼������Һ
��5��ʵ������ռ���ˮ���������ˮ�����������Ϊ2.70g���������ϩ������IJ���ԼΪ85.2%��ʵ���м���ϩ�������ʵ�ʲ�������С�ڴ˼���ֵ����ԭ������c��
a����ˮ���ռ���ˮ�ﺬ����
b��ʵ�������·�������Ӧ
c����Ʒ����ʱ�ռ����ֵͷе�����
d����Ʒ��ϴ�ӡ���������������ʧ��
���� ��1��װ��AΪ���������ܣ�
��2��Ũ�������ղ���ˮ��ʹ������Ӧ���������ƶ���Ũ�������ǿ�����ԣ��������л���Ӧ�
��3���״�֮����Է������Ӽ���ˮ��Ӧ���ɼ��ѣ�
��4�����Լ�Xϴ�Ӳ�Ʒ����Ҫϴȥ�������ἰ�״�����Ӧ���������ܽ�ȣ��������������ϴ�ӽ��
��5���������ϩ�ᡢ�״������������й������㣬���ݲ����������ʼ����Ʒ�����۲�������������ˮ�����������Ʒʵ�ʲ���������������ʣ�
a����ˮ���ռ���ˮ�ﺬ��������Ʒ������ƫС��
b��ʵ�������·�������Ӧ���ᵼ�²�Ʒ����ƫС��
c����Ʒ����ʱ�ռ����ֵͷе����ʣ��ᵼ�²�Ʒ����ƫ��
d����Ʒ��ϴ�ӡ���������������ʧ���ᵼ�²�Ʒ����ƫС��
��� �⣺��1���������Ľṹ��������֪װ��AΪ���������ܣ��ʴ�Ϊ�����������ܣ�
��2��Ũ�������ղ���ˮ����ʹ������Ӧ���������ƶ������������ϩ��������ʣ�Ũ�������ǿ�����ԣ��������л���Ӧ�Ũ������࣬���ʷ������ͣ�
�ʴ�Ϊ��Ũ�������ղ���ˮ����ʹ������Ӧ���������ƶ���
��3���״�֮����Է������Ӽ���ˮ��Ӧ���ɼ��ѣ����ܲ����ĸ�������CH3OCH3�ȣ�
�ʴ�Ϊ��CH3OCH3��
��4�����Լ�Xϴ�Ӳ�Ʒ����Ҫϴȥ�������ἰ�״�����Ӧ���������ܽ�ȣ��������������ϴ�ӿ�֪��ѡ��̼������Һ������ʳ��ˮ����ȥ���ᣬ����������Һ����̫ǿ���ᵼ�²�Ʒˮ�⣬
�ʴ�Ϊ��c��
��5������ϩ�ᡢ15mL����ϩ�������Ϊ15mL��1.01g/mL=15.15g��10mL��ˮ�״�������Ϊ10mL��0.79g/mL=7.9g���״���ȫ��Ӧ��Ҫ����ϩ�������Ϊ$\frac{7.9g}{32g/mol}$��86g/mol=21.23g��15.15g���ʼ״����������������ɼ���ϩ�����������Ϊ$\frac{15.15g}{86g/mol}$��100g/mol��
�������ˮ������Ϊ2.70g����ʵ�ʵõ�����ϩ�����������Ϊ$\frac{2.7g}{18g/mol}$��100g/mol=15g��
�ʲ���Ϊ[15g�£�$\frac{15.15g}{86g/mol}$��100g/mol��]��100%=85.2%��
a����ˮ���ռ���ˮ�ﺬ��������Ʒ������ƫС������ƫ�ͣ���a��ѡ��
b��ʵ�������·�������Ӧ���ᵼ�²�Ʒ����ƫС������ƫ�ͣ���b��ѡ��
c����Ʒ����ʱ�ռ����ֵͷе����ʣ��ᵼ�²�Ʒ����ƫ����ƫ��cѡ��
d����Ʒ��ϴ�ӡ���������������ʧ���ᵼ�²�Ʒ����ƫС������ƫ�ͣ���d��ѡ��
�ʴ�Ϊ��85.2%��c��
���� ���⿼���л�����Ʊ�ʵ�飬�漰��ѧ����ʶ�����ʵķ����ᴿ��ƽ���ƶ������ʼ���ȣ�ע�����������������Ʊ�����Ǩ�ƽ��

| ѡ�� | ���� | ���� |
| A | Cl2��SO2�ֱ�ͨ����ɫʯ����Һ�У���Һ����ɫ | ���߾���Ư���� |
| B | CO2��SO2�ֱ�ͨ��Ba��NO3��2��Һ�У����������� | �������ǿ�� |
| C | ����Һ�еμ����ᣬ����������ͨ�����ʯ��ˮ�У�ʯ��ˮ����� | ����Һ��һ����CO32- |
| D | ���ء��Ʒֱ����װ��ú�͵��Թ��У����߾��³����Թܵײ� | �ء��Ƶ��ܶȶ���ú�ʹ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
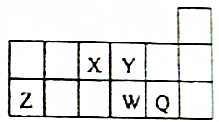 ������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������
������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������| A�� | Ԫ��X����ͻ��ϼ۾���ֵ��Ԫ��X����������ϼ�֮�͵���ֵ����8 | |
| B�� | �����Ӱ뾶�Ĵ�С˳��Ϊ��Z3+��W2-��Q-��Y2- | |
| C�� | Y����Ԫ���γɵĻ������о�ֻ�����Թ��ۼ� | |
| D�� | ��۵�X��Q�γɵĻ������У���ԭ�Ӷ�Ϊ8�����ȶ��ṹ |
 NH2+CHCOOH$\stackrel{��}{?}$
NH2+CHCOOH$\stackrel{��}{?}$
 +H2O
+H2O| ���� | ��Է������� | ��״ | �ܶ�/��g/cm3�� | �۵�/�� | �е�/�� | �ܽ�� | |
| ���� | 93 | ��ɫ��״Һ����л�ԭ�� | 1.02 | -6.1 | 184 | ����ˮ | ���������Ҵ������� |
| ���� | 60 | ��ɫҺ�� | 1.05 | 16.5 | 118 | ������ˮ | |
| ���� ���� | 135 | ��ɫ���� | 1.22 | 114 | 304 | ������ˮ��������ˮ | |
��50mlԲ����ƿ�м�����ˮ����5ml����������7.5mL��п��0��lg������ͼ��װ�����������ʯ������Ӧ�����ȼ��ȣ�ʹ��ӦҺ����״̬�»��������ڼ����¶ȣ�ʹ�����¶ȿ�����105�����ң���ӦԼ60��80nun������Ӧ�������ʱ��ֹͣ���ȣ�
�ڽ����£����Ƚ���ƿ�е����ϵ���ʢ��l00mL��ˮ���ձ��У����ҽ��裬����ȴ�ձ������£������������ᾧ���������ˡ�ϴ�ӡ�����õ�����������Ʒ������Ʒ�ؽᾧ�����ˣ����ɣ����أ�������ʣ�
ע��DΪ���η����������ڷе���̫��Ļ����ķ��룮
 ��
����ش��������⣺
��1������A�����������ܣ�
��2��װ��ͼ�м��ȿ�����ԡ���ˮԡ������ԡ������
��3��ʵ���м���п�۵�Ŀ���Ƿ�ֹ�����ڷ�Ӧ�����б�������
��4��Ϊ��Ҫ���Ʒ������϶˵��¶���105�����Ҳ��Ϸֳ���Ӧ���������ɵ�ˮ���ٽ���Ӧ������У����������IJ��ʣ�
��5��ͨ���۲쵽�¶ȼ��¶��½�����ƿ��Һ�岻�����ӣ�������жϷ�Ӧ������ɣ���Ӧ�����������������������õı�ˮ�е�ԭ�������������۵�ϸߣ����伴��̻�����������ƿ����������
��6��ϴ������������Ʒ����ʵ��Լ���a��
a����������ˮϴ b����������ˮϴc��������ˮϴ��������ˮϴ d���þƾ�ϴ
��7����ʵ�����յõ���Ʒ1.8g�������������IJ�����24%��
| A�� | ��10 mL0.1 mol•L-1Na2CO3��Һ��εμӵ�10 mlL0.1 mol•L-1�����У������Һ�и�����Ũ�ȵĴ�С��ϵ��c��Na+����c��Cl-����c��HCO3-����c��CO32-�� | |
| B�� | ���ʵ���Ũ����ȵ�NaF��Һ��CH3COONa��Һ��Ƚϣ��������ӵ���Ũ����� | |
| C�� | ��0.1mol•L-1��FeCl3��Һ�еμ���������KMnO4��Һ��KMnO4��Һ����ɫ��˵��FeCl3ֻ�������ԣ��������������� | |
| D�� | ��AgCl��AgBr�ı�����Һ�������ϣ��ټ�������AgNO3Ũ��Һ���ɹ۲쵽��������ɫ������������ɫ������˵��Ksp��AgCl����Ksp��AgBr�� |
| A�� | KNO3 | B�� | C | C�� | KNO3��S | D�� | N2��CO2 |
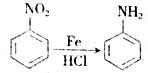
 ������A�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
������A�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��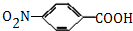 $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$ +H2O��
+H2O�� �ȣ�
�ȣ�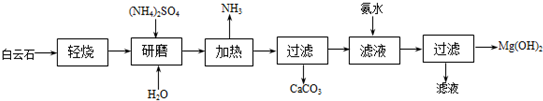
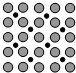 ��Ӧ���ںϳɰ���Ӧ�Ĵ����������ı����ϴ��ڵ�ԭ�ӣ���ͼΪ��ԭ�������ľ����ϵĵ��㸽�žֲ�ʾ��ͼ��ͼ��С��ɫ�������ԭ�ӣ���ɫ�������ԭ�ӣ�����ͼʾ�����������ϵ�ԭ������ԭ�ӵĸ�����Ϊ1��2��
��Ӧ���ںϳɰ���Ӧ�Ĵ����������ı����ϴ��ڵ�ԭ�ӣ���ͼΪ��ԭ�������ľ����ϵĵ��㸽�žֲ�ʾ��ͼ��ͼ��С��ɫ�������ԭ�ӣ���ɫ�������ԭ�ӣ�����ͼʾ�����������ϵ�ԭ������ԭ�ӵĸ�����Ϊ1��2��