��Ŀ����
4��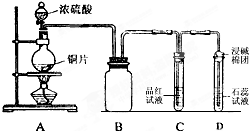 ijͬѧΪ̽��ͭ��Ũ����ķ�Ӧ���������ͼ��ʾװ�ý������й�ʵ�飮
ijͬѧΪ̽��ͭ��Ũ����ķ�Ӧ���������ͼ��ʾװ�ý������й�ʵ�飮��1��B�������ռ�ʵ�������ɵ������װ�ã���δ�����ܻ�ȫ�����ڴ�����ϰѵ��ܲ�������
 ��
����2����д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ2H2SO4��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2H2O+2SO2����
��3��ʵ����D�е�������ɫʯ����Һ��죮
��4��ʵ���У���ͬѧȡ6.4gͭƬ��12mL 18mol•L-1 H2SO4��Һ����Բ����ƿ�й��ȣ�ֱ����Ӧֹͣ���������ƿ�л���ͭƬʣ�ࣻ��ͬѧ������ѧ�Ļ�ѧ֪ʶ�жϻ�����һ����H2SO4��ʣ�࣬����ҩƷ���ܹ�������֤��Ӧֹͣ�����ƿ��ȷ��ʣ���H2SO4����bd����ĸ��ţ���
a��BaCl2��Һ b��Ba��NO3��2��Һ c������ d��Na2CO3��ĩ
��5������16gͭ��50mL H2SO4���ʵ���Ũ��Ϊһ��ֵ��Ũ���ᷴӦ��ͭ��ȫ�ܽ⣮��ش�
�ٷ�Ӧ�в����������ڱ�״���µ����Ϊ5.6L��
�ڸ÷�Ӧ�б���ԭ��H2SO4�����ʵ���Ϊ0.25mol��
�۴�����������ȫ���ͷź�����Һ�еμ�V mL a mol•L-1 NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת��Ϊ��������ԭŨ������H2SO4�����ʵ���Ũ��=��aV��10-2+5��mol•L-1��
���� ��1��ͭ��Ũ������ȷ�Ӧ���ɶ����������壬����������ܶȴ��ڿ������ռ�ʱӦ�ý����ܳ������̣ܶ��ݴ˻����ռ�װ�ã�
��2��ͭ��Ũ������ȷ�Ӧ��������ͭ�����������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��3����������Ϊ�����������ˮ��Һ��ʾ���ԣ��ܹ�ʹ��ɫʯ����Һ��죻
��4������ϡ����Ĵ��ڣ�ֻ����鷴Ӧ�����Һ�д��������ӵļ��ɣ�ע�����ᱵ�к�����������ӣ�����������������������ܹ�����ͭ��
��5���ٸ���n=$\frac{m}{M}$�����ͭ�����ʵ������ٸ��ݷ�Ӧ���������¶�����������ʵ������ټ��������¶�������������
��ͭ��Ũ����ķ�Ӧ�У�Ũ����Ϊ����������Ӧ�б������ɶ��������������������ʵ�����Ϊ����ԭ����������ʵ�����
��ͭ���ӷ�Ӧ����������ͭʱ��ʣ��������������ȫת���������ƣ������������Ƶ����ʵ��������ʣ����������ӵ����ʵ�����ԭ50mLŨ�����к��е���������ʵ���Ϊ������������ʵ�����ʣ����������ӵ�֮�ͣ�������c=$\frac{n}{V}$�����Ũ�ȣ�
��� �⣺��1��B�������ռ�ʵ�������ɵ������װ�ã�������Ϊ�����������ڶ���������ܶȴ��ڿ������ռ�ʱӦ�ý����ܳ������̣ܶ������ռ����������װ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ���������������ˮ����Ӧ�����ӷ���ʽΪ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��3��������������ˮ���������ᣬ������Ϊ���ᣬ�ܹ�����������ӣ�����Һ��ʾ���ԣ������Թ�D�е���ɫʯ����Һ���죬
�ʴ�Ϊ����ɫʯ����Һ��죻
��4��ϡ������ǿ����������ϡ��Һ������������Ӻ���������ӣ�����Ϊ֤����Ӧ���������ƿ��ȷ�����ᣬ����������ӣ�
a������BaCl2��Һ����������ӷ�Ӧ���ɳ���������ʣ��ʱ����Һ�д�����������ӣ���֤�������Ӵ��ڣ����ܼ��飬��a����
b������������������������ܹ�����ͭ��������Ba��NO3��2��Һ��ͭƬ�����ܽ��Ҳ������壬˵����Ӧ����Һ�к��������ӣ���֤��������ʣ�࣬��b��ȷ��
c������������������֮���ܹ���ϡ���ᷴӦ�������飬��c����
d��Na2CO3��Һ�������̼������Ӻ������ӷ�Ӧ���ɶ�����̼��ˮ�������ܼ��飬��d��ȷ��
�ʴ�Ϊ��bd��
��5����16gͭ�����ʵ���Ϊ��$\frac{16g}{64g/mol}$=0.25mol��ͭ��ȫ��Ӧ�����ݷ�ӦCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��֪��0.25molͭ��ȫ��Ӧ������0.25mol�����������0.25mol������������Ϊ��22.4L/mol��0.25mol=5.6L��
�ʴ�Ϊ��5.6��
�ڸ��ݻ��ϼ۱仯��֪���÷�Ӧ��Ũ���ᱻ��ԭ���ɶ�����������������ʵ����뱻��ԭ����������ʵ�����ȣ���ԭ��H2SO4�����ʵ���Ϊ0.25mol��
�ʴ�Ϊ��0.25��
��V mL a mol•L-1 NaOH��Һ�к����������Ƶ����ʵ���Ϊ��amol/L��0.001VL=0.001aVmol��
ͭ������ȫ����ʱ����Ӧ������Ϊ�����ƣ���Ӧ����Һ��ʣ����������ӵ����ʵ���Ϊ��$\frac{1}{2}$��0.001aVmol=0.0005aVmol��
��ԭ50mLŨ�����к�����������ʵ���Ϊ��0.0005aVmol+0.25mol��
����ԭŨ��������ʵ���Ũ��Ϊ��$\frac{0.0005aVmol+0.25mol}{0.05L}$=��aV��10-2+5��mol/L��
�ʴ�Ϊ����aV��10-2+5����
���� ���⿼����Ũ��������ʡ���ѧ����ʽ�ļ��㣬��Ŀ�Ѷ��еȣ�ע������ͭ��Ũ����ķ�Ӧԭ������ȷ���ʵ����ڻ�ѧ����ʽ�ļ����е�Ӧ�ã�������ؿ���ѧ���ķ�����������ѧʵ�顢��ѧ����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ���³�ѹ�£�22.4 L��ϩ�к�C-H������ĿΪ4NA | |
| B�� | 0.1 mol•L-1�ģ�NH4��2SO4��Һ�к��������ӵ�����Ϊ0.2NA | |
| C�� | 7.8 g Na2S�����7.8 g Na2O2�����к��е���������Ŀ��Ϊ0.1NA | |
| D�� | ��״���£�2.24 L Cl2�����ϡNaOH��Һ��Ӧ��ת�Ƶĵ�������Ϊ0.2NA |
| Ԫ�� | H | Li | Be | B | C | N | O | F |
| �縺�� | 2.1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 0.8 |
��1�����ݱ����������ݷ����Ʋ⣺
ͬ����IJ�ͬԪ�صĵ縺�Ա仯�Ĺ����ǣ����϶��µ縺�Խ���
ͬ�����У��縺����ԭ�Ӱ뾶�Ĺ�ϵΪ��ԭ�Ӱ뾶ԽС��Ԫ�ص縺��Խ��
��2��Ԥ�����ڱ��е縺������Ԫ��ӦΪF����Ԫ�ط��ţ������Ƹ�Ԫ�صĵ縺�Ե�ȡֵ��Χ��0.8��Ca��1.2
��3��Ԥ�����ڱ��У��縺����С��Ԫ��λ�ڵ������ڢ�A�壨������Ԫ�س��⣩

| A�� | ��̪���ڷ����� | |
| B�� | ��̪�ķ���ʽΪC20H12O4 | |
| C�� | ��̪�ṹ�к����ǻ���-OH�����ʷ�̪���ڴ� | |
| D�� | ��̪�ڼ����������ܹ�����ˮ�ⷴӦ |
�ٳ�ȥ��������������ϩ����������ͨ��H2���ڳ�ȥ��������������������ñ���̼��������Һϴ�ӣ���Һ��������۳�ȥC2H4��������SO2������ͨ��ʢ����������Һ��ϴ��ƿ���ܳ�ȥ�Ҵ���������ˮ����������ʯ�ң�����
| A�� | �٢ڢ� | B�� | �ڢ� | C�� | �٢ۢ� | D�� | �ۢ� |
��1�����������ҺӦ���������Һ����������ɫ�Լ�ƿ�У�����ע����������������¸��������Һ�ֽ�����ӷ���ʽ4MnO4-+4H+�T4MnO2��+3O2��+2H2O��
��2������ƽ���������Һ�������Na2C2O4��Һ�����������·�Ӧ�����ӷ���ʽ��MnO4-+C2O42-+H+--Mn2++CO2��+8H2O
��3��ijѧϰС��Ϊ��̽�����������Һ�Ͳ�������Һ�ķ�Ӧ���̣������������Һ��εص���һ����������Բ�������Һ�У��¶���ͬ����������ʱ������¼�����������
������������Һ�Ĵ���ÿ����Һ�������ͬ�� | ���������Һ��ɫ��ȥ��ʱ�� |
| �ȵ����1�� | 1min |
| ��ɫ���ٵ����2�� | 15s |
| ��ɫ���ٵ����3�� | 3s |
| ��ɫ���ٵ����4�� | 1s |
��4����ѧϰС���ڻ�ȡ����������ͽ����Ժ����ȶ������ʲ�����Na2C2O4����Է�������134.0�����궨���������Һ��Ũ�ȣ�����ȷ��ȡ1.340g�����IJ��������250mL��Һ��ÿ��ȷ��ȡ25.00mL��Һ�ữ����KMnO4��Һ�ζ���
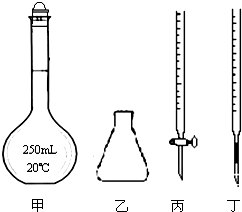
�ٸ��������ҺӦװ�ڱ�������ͼ�е�������ţ���
��Ϊ�˷�ֹ������������������·ֽ��������ζ�ʱӦע�������ε�����������Һ����ǰһ�θ��������Һ��ɫ��ȥ���ٵμӣ�
������ʵ������д������в��������л�ʹ����KMnO4Ũ��ƫ�͵���A��
A��δ��ϴʢ��KMnO4�ĵζ���
B���ζ�ǰ���첿�������ݣ��ζ��յ�ʱ��������
C������ʱ�����ӿ̶���
D����ƿ��ˮϴ֮��δ�ô���Һ��ϴ
�ܵ���Һ����ɫ�Ұ�����ڲ���ɫ������KMnO4��Һ20.00mL����βⶨ��ƽ��ֵ������KMnO4��Һ��Ũ��Ϊ0.02mol/L������ԭ���������K=39 Mn=55 O=16 Na=23 C=12��
| X | ||||
| Y | Z | W |
| A�� | X�ֱ���Y��Z�γɵĻ������л�ѧ��������ͬ | |
| B�� | Z�����������Ķ�Ӧˮ�������Ա�W��ǿ | |
| C�� | X�ļ���̬�⻯����ȶ��Ա�W��ǿ | |
| D�� | ԭ�Ӱ뾶�Ĵ�С˳��r��Y����r��Z����r��W����r��X�� |
| A�� | NH4HSO4��Һ�еμ�NaOH��Һ��pH=7����c��Na+��=2c��SO42-�� | |
| B�� | 0.1 mol•L-1CH3COOH��Һ��0.1 mol•L-1CH3COONa��������pH��7����c��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-�� | |
| C�� | AgCl��ˮ���ܽ������ϡ�������ܽ����ͬ | |
| D�� | KNO3��Һ��CH3COONH4��ҺpH��Ϊ7��������Һ��ˮ�ĵ���̶���ͬ |
| A�� | Ԫ��A��B��ɵĻ����ﳣ����һ������̬ | |
| B�� | һ�������£�Ԫ��C��D������������Ӧ��ˮ����֮���ܷ�����Ӧ | |
| C�� | ��ҵ�ϳ��õ�ⷨ�Ʊ�Ԫ��A��C��D�ĵ��� | |
| D�� | ������AE��CE������ͬ���͵Ļ�ѧ�� |