题目内容
5.(1)氨催化氧化法是工业制硝酸的主要方法,可进行连续生产.已知:N2(g)+O2(g)=2NO(g)△H=+180.5kJ/mol
N2(g)+3H2(g)?2NH3(g)△H=-92.4kJ/mol
2H2(g)+O2(g)=2H2O(g)△H=-483.6kJ/mol
写出氨气经催化氧化生成一氧化氮气体和水蒸气的热化学方程式:4NH3(g)+5O2(g)═4NO(g)+6H2O(g)△H=-905.0kJ/mol.
(2)恒容密闭容器中进行的合成氨反应,其化学平衡常数K与温度t的关系如下表:
| t/K | 298 | 398 | 498 | … |
| K | 4.1×106 | K1 | K2 | … |
②上表中K1>K2(填“>”、“=”或“<”).
(3)如果向氨合成塔中充入10molN2和40molH2进行氨的合成,图A和图B为一定温度下平衡混合物中氨气的体积分数与压强(p)的关系图.
①下列说法正确的是ABD(填序号).
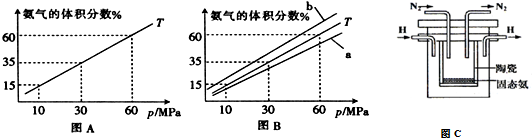
A.图中曲线表明增大体系压强(p),有利于提高氨气在混合气体中体积分数
B.如果图B中T=500℃,则温度为450℃时对应的曲线是b
C.工业上采用500℃温度可有效提高反应速率和氮气的转化率
D.当 2v正(H2)=3v逆(NH3)时,反应达到平衡状态
E.容器内混合气体密度保持不变时,反应达到平衡状态
②图A中氨气的体积分数为15%时,N2的转化率为32.61%.
(4)在1998年希腊亚里斯多德大学的Marmellos和Stoukides采用高质子导电性的SCY陶瓷(能传递H+),实现了高温常压下高转化率的电化学合成氨,其实验装置如图C,则正极的电极反应式N2+6e-+6H+=2NH3.
(5)25℃时,Ksp[Mg(OH)2]=5.61×10-12,Ksp[MgF2]=7.42×10-11.下列说法正确的是BD.
A.25℃时,饱和Mg(OH)2溶液中c(OH-)大于饱和MgF2溶液中c(F-)
B.25℃时,某饱和Mg(OH)2溶液中c(Mg2+)=0.0561 mol•L-1,则溶液的pH=9
C. 25℃时,在Mg(OH)2的悬浊液中加入少量的NH4Cl固体,溶液变澄清,Ksp[Mg(OH)2]增大
D.25℃时,在Mg(OH)2悬浊液中加入NaF溶液后,Mg(OH)2可能转化为MgF2.
分析 (1)根据盖斯定律,由已知热化学方程式乘以适当的系数进行加减构造目标热化学方程式,反应热也进行相应的计算;
(2)①化学平衡常数,是一定温度下,可逆反应到达平衡,生成物浓度化学计量次数幂之积与反应物浓度化学计量次数幂之积所得的比值;
②合成氨是放热反应,升高温度,平衡向逆反应方向移动,化学平衡常数减小;
(3)①A.由图可知,温度一定时,压强增大氨气的体积分数增大;
B.合成氨是放热反应,压强一定时,降低温度平衡向正反应方向移动,平衡时氨气的体积分数增大;
C.500℃温度时反应速率加快及催化剂活性最好,正反应为放热反应,温度越低,氮气的转化率越高;
D.不同物质表示的正逆速率之比等于化学计量数之比,说明可逆反应到达平衡;
E.容器内混合气体密度始终保持不变;
②设参加反应的氮气为n mol,则:
N2(g)+3H2(g)?2NH3(g)
开始(mol):10 40 0
转化(mol):n 3n 2n
平衡(mol):10-n 40-3n 2n
根据平衡时氨气的体积分数方程式计算n,进而计算氮气的转化率;
(4)阴极发生还原反应,氮气在阴极上放电,与氢离子结合生成氨气;
(5)A.氢氧化镁与氟化镁的化学式相似,氢氧化镁的溶度积小,溶液中氢氧化镁的浓度小于氟化镁;
B.根据镁离子浓度、氢氧化镁溶度积计算溶液中c(OH-),再根据水的离子积计算溶液中c(H+),再根据pH=-lgc(H+)计算;
C.溶度积Ksp不随浓度变化,只与温度有关;
D.不管氢氧化镁的ksp有多小,只要加入的氟化钠溶液的浓度适合的话,使c(Mg2+)×c(F-)2>7.42×10-11,可以使氢氧化镁转化为氟化镁沉淀.
解答 解:(1)已知:①N2(g)+O2(g)=2NO(g)△H=+180.5kJ/mol
②N2(g)+3H2(g)?2NH3(g)△H=-92.4kJ/mol
③2H2(g)+O2(g)=2H2O(g)△H=-483.6kJ/mol
由盖斯定律可知,①×2-②×2+③×3得:4NH3(g)+5O2(g)═4NO(g)+6H2O(g)△H=(+180.5kJ/mol)×2-(-92.4kJ/mol)×2+(-483.6kJ/mol)×3=-905.2kJ/mol,
故答案为:4NH3(g)+5O2(g)═4NO(g)+6H2O(g)△H=-905.0kJ/mol;
(2)①N2(g)+3H2(g)?2NH3(g)的平衡常数表达式K=c2(NH3)c(N2)×c3(H3),
故答案为:c2(NH3)c(N2)×c3(H3);
②合成氨反应N2(g)+3H2(g) 2NH3(g)△H<0,对于放热反应,温度越高,越有利于向逆反应进行,所以K1>K2,
2NH3(g)△H<0,对于放热反应,温度越高,越有利于向逆反应进行,所以K1>K2,
故答案为:>;
(3)①A.由图可知,温度一定时,压强增大氨气的体积分数增大,故A正确;
B.图B中T=500℃,合成氨是放热反应,压强一定时,降低温度平衡向正反应方向移动,平衡时氨气的体积分数增大,则温度为450℃时对应的曲线是b,故B正确;
C.500℃温度时反应速率加快及催化剂活性最好,正反应为放热反应,温度越低氮气的转化率越高,高温不利于氮气的转化,故C错误;
D.当 2v正(H2)=3v逆(NH3),不同物质表示的正逆速率之比等于化学计量数之比,说明可逆反应到达平衡,故D正确;
E.容器内混合气体密度始终保持不变,不能说明到达平衡,故E错误,
故选:ABD;
②设参加反应的氮气为n mol,则:
N2(g)+3H2(g)?2NH3(g)
开始(mol):10 40 0
转化(mol):n 3n 2n
平衡(mol):10-n 40-3n 2n
则2n10−n+40−3n+2n×100%=15%,解得n≈3.261,所以氮气的转化率为3.261mol10mol×100%=32.61%,
故答案为:32.61%;
(4)阴极发生还原反应,氮气在阴极上放电,与氢离子结合生成氨气,电极反应式为N2+6e-+6H+=2NH3.
故答案为:N2+6e-+6H+=2NH3;
(5)A.氢氧化镁与氟化镁的化学式相似,氢氧化镁的溶度积小,溶液中氢氧化镁的浓度小于氟化镁,饱和Mg(OH)2溶液中c(OH-)小于饱和MgF2溶液中c(F-),故A错误;
B.饱和Mg(OH)2溶液中c(Mg2+)=0.0561 mol•L-1,Ksp[Mg(OH)2]=5.61×10-12,则溶液中c(OH-)=1×10-5mol/L,故溶液中溶液中c(H+)=1×10-9mol/L,溶液pH=-lgc(H+)=9,故B正确;
C.溶度积Ksp不随浓度变化,只与温度有关,故C错误;
D.只要加入的氟化钠溶液的浓度适合的话,使c(Mg2+)×c(F-)2>7.42×10-11,可以使氢氧化镁转化为氟化镁沉淀,故D正确,
故选:BD.
点评 本题考查热化学方程式书写、平衡常数及有关计算、化学平衡计算、化学平衡图象及影响因素、原电池、溶度积等,属于拼合型题目,需要学生具备扎实的基础,难度中等.

①乙烷(乙烯):光照条件下通入Cl2,气液分离
②乙炔(硫化氢):酸性高锰酸钾溶液,洗气
③苯(苯酚):用浓溴水洗涤,分液
④乙醇(乙酸):加足量生石灰,蒸馏.
| A. | 都不正确 | B. | ②③ | C. | ④ | D. | ③④ |
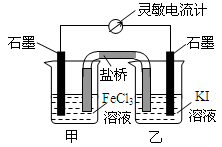
| A. | 反应开始时,甲池中电极反应为Fe3++e-=Fe2+ | |
| B. | 反应开始时,盐桥中的阴离子向乙池迁移 | |
| C. | 反应处于化学平衡状态时,甲、乙两池中离子浓度不再变化 | |
| D. | 反应处于化学平衡状态时,电子沿着石墨(乙池)→电流计→石墨(甲池)路径流动 |
 某学习小组探究NaHCO3、Na2HCO3和盐酸(盐酸浓度均为1mol.L-1)反应过程中的热效应,实验测得如下数据:
某学习小组探究NaHCO3、Na2HCO3和盐酸(盐酸浓度均为1mol.L-1)反应过程中的热效应,实验测得如下数据:| 序号 | 35mL试剂 | 固体 | 混合温度前/℃ | 混合温度后/℃ |
| ① | 水 | 2.5gNaHCO3 | 20.0 | 18.5 |
| ② | 水 | 3.2gNa2CO3 | 20.0 | 24.3 |
| ③ | 盐酸 | 2.5gNaHCO3 | 20.0 | 16.2 |
| ④ | 盐酸 | 3.2gNa2CO3 | 20.0 | 25.1 |
(1)写出NaHCO3和盐酸发生反应的离子方程式HCO3-+H+=CO2↑+H2O
(2)由上述实验得出的结论是:Na2CO3溶液与盐酸的反应是放热(填“吸热”或“放热”下同)反应,NaHCO3溶液与盐酸反应是吸热反应
(3)在如图中画出Na2CO3和盐酸反应前后能量变化曲线(标注“反应物总能量”和“生成物总能量”)
| A. | 0.05mol•L-1 | B. | 0.1 mol•L-1 | C. | 0.5 mol•L-1 | D. | 1 mol•L-1 |
| A. | CuO | B. | H2SO4 | C. | CuSO4 | D. | Al |
| A. | 该氨水显弱碱性 | |
| B. | 加水稀释过程中,c(H+)/c(OH-)的值减小 | |
| C. | 与同温下pH=11的NaOH溶液相比,NaOH溶液中c(Na+)大于氨水中c(NH4+) | |
| D. | 加入少量NH4Cl 固体,溶液中水的电离平衡:H2O?H++OH-向右移动 |