��Ŀ����
14����֪���ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ���ἰ���ι㷺����ҽҩ��ӡȾ�����ϵȹ�ҵ����1����֪25��ʱ�����ֳ��������Ka���±���ʾ��
| ����� | H2C2O4 | CH3COOH | HCN | H2CO3 |
| ���볣����mol•L-1�� | K1=5.6��10-2 K2=5.4��10-3 | K1=1.7��10-5 | K2=6.2��10-10 | K1=4.2��10-7 K2=5.6��10-11 |
�����й���0.1mol•L?1NaHC2O4��Һ��˵����ȷ����ad��
a��HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ���Һ������
b��HC2O4-��ˮ��̶ȴ��ڵ���̶ȳ̶ȣ���Һ�Լ���
c����Һ��c��Na+��+c��H+��=c��HC2O4-��+c��OH-��+c��C2O42-��
d����Һ��c��H+��=c��OH-��+c��C2O42-��-c��H2C2O4��
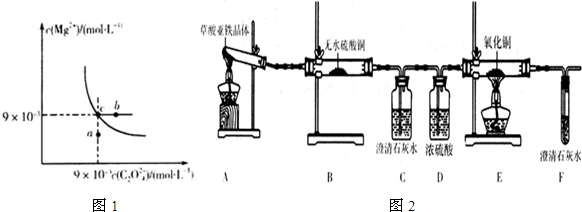
��2����t��ʱ��MgC2O4��ˮ�еij����ܽ�ƽ��������ͼ1��ʾ����֪t��ʱMg��OH��2��Ksp=5.6��10-12������˵������ȷ����BD��
A����t��ʱ��MgC2O4��Ksp=8.1��10-5��mol•L-1����
B����MgC2O4������Һ�м���Na2CO3���壬��ʹ��Һ��c�㵽b��
C��ͼ��a���Ӧ����MgC2O4�IJ�������Һ
D����t��ʱ��MgC2O4 ��s��+2OH-��aq��?Mg��OH��2��s��+C2O42-��aq��ƽ�ⳣ��K=Ksp[Mg��OH��2]/Ksp ��MgC2O4��
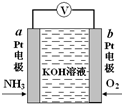
��3�������������壨FeC2O4•2H2O����һ��dz��ɫ���壬������ˮ�������ֽ⣮ij��ѧ��ȤС�����ʵ����֤�������������ȷֽ�IJ��̽��������ͼ2��
�ٴӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����ȱ��β������װ�ã�
�ڸ���ȤС���������ʵ��ʱ����ʵ�鰲ȫ�Ƕȿ��ǣ�Ӧ�ȵ�ȼA���A����E�������ľƾ��ƣ�
����ʵ������й۲쵽B�а�ɫ��ˮCuSO4�����ɫ��C�г���ʯ��ˮ����ǣ�E�к�ɫ��ĩ���ɫ��������ʵ���������֤���������������ȷֽ�����������H2O��CO��CO2��
��Ϊ̽��������������ֽ�Ĺ�������ȤС��ͬѧȷ��ȡ3.60g�����������壨FeC2O4•2H2O������Է���������180������ּ��ȣ�ʹ����ȫ�ֽ⣬��ȴ��Ƶ�ʣ����������Ϊ1.60g����ʣ�����ֻ��һ�����������ͨ������ȷ����������Ļ�ѧʽΪFe2O3��������������ֽ�Ļ�ѧ����ʽΪFeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe2O3+3CO��+CO2��+2H2O��
���� ��1���ٵ��볣��Խ���������Խǿ��������Һ��ˮ��̶�ԽС������Һ��pHԽС��pH��ͬʱ������Խǿ�������Ũ��ԽС��
����֪K2=$\frac{c��{C}_{2}{{O}_{4}}^{2-}����c��{H}^{+}��}{c��H{C}_{2}{{O}_{4}}^{-}��}$=5.4��10-3����HC2O4-��Һ�����ԣ���ϵ���غ�������غ������
��2��A��MgC2O4��Ksp=c��Mg2+����c��C2O42-�������ͼ�����ݼ��㣻
B����MgC2O4������Һ�м���Na2CO3���壬������̼��þ������
C�������ܽ�ƽ����������ĵ������������Һ��
D�����ݷ�Ӧ��ƽ�ⳣ������ʽ��Ksp�ı���ʽ������
��3����CO�����ж����壬Ҫ����β��������
�ڿ�ȼ��������������ϴﵽһ���̶�ʱ��������ᷢ����ը�������ȵ�ȼ�ֽⷴӦ���ľƾ��ƣ��ž�װ���еĿ�����
�۶�����̼�����ʯ��ˮ��Ӧ����̼��Ƴ�����CO��ԭCuO�õ�Cu��
�ܸ��ݲ�����������������������FeԪ�ص���������Ԫ���غ��֪��������Fe�������������OԪ�ص�������Ȼ���������������Ļ�ѧʽ�����������ṩ����Ϣ����֪������������ֽ�IJ���Ϊ߯�������H2O��CO��CO2������ԭ���غ�д������ʽ��
��� �⣺��1���ٵ��볣��Խ���������Խǿ��������Һ��ˮ��̶�ԽС������Һ��pHԽС����Ũ�Ⱦ�Ϊ0.1mol•L-1��Na2C2O4��CH3COONa��NaCN��Na2CO3��pH�ɴ�С��˳����Na2CO3��NaCN��CH3COONa��Na2C2O4��pH��ͬʱ������Խǿ�������Ũ��ԽС����֪��������Դ���HCN����pH��ͬʱHCN��Ũ�ȴ������к͵��������pH��HCOOH��HCN����NaOH������HCN���ĵ��������ƶࣻ
�ʴ�Ϊ��Na2CO3��NaCN��CH3COONa��Na2C2O4�����ߴ�
��a����֪K2=$\frac{c��{C}_{2}{{O}_{4}}^{2-}����c��{H}^{+}��}{c��H{C}_{2}{{O}_{4}}^{-}��}$=5.4��10-3����HC2O4-��Һ�����ԣ���HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ���a��ȷ��
b��HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ���b����
c��NaHC2O4��Һ���ڵ���غ㣬����Һ��c��Na+��+c��H+��=c��HC2O4-��+c��OH-��+2c��C2O42-������c����
d��NaHC2O4��Һ���ڵ���غ�Ϊc��Na+��+c��H+��=c��HC2O4-��+c��OH-��+2c��C2O42-���������غ�Ϊ��c��Na+��=c��HC2O4-��++c��C2O42-��+c��H2C2O4������������ʽ�ӿɵã�c��H+��=c��OH-��+c��C2O42-��-c��H2C2O4������d��ȷ��
�ʴ�Ϊ��ad��
��2��A��MgC2O4��Ksp=c��Mg2+����c��C2O42-��=��9��10-3��2=8.1��10-5��mol•L-1������A��ȷ��
B����MgC2O4������Һ�м���Na2CO3���壬������̼��þ��������þ����Ũ�ȼ�С��ͼ����c�㵽b��ʱþ����Ũ�Ȳ��䣬��B����
C�������ܽ�ƽ����������ĵ������������Һ����a���Ӧ����MgC2O4�IJ�������Һ����C��ȷ��
D��MgC2O4 ��s��+2OH-��aq��?Mg��OH��2��s��+C2O42-��aq��ƽ�ⳣ��K=$\frac{c��{C}_{2}{{O}_{4}}^{2-}��}{{c}^{2}��O{H}^{-}��}$=$\frac{Ksp[Mg{C}_{2}{O}_{4}]}{Ksp[Mg��OH��_{2}]}$����D����
�ʴ�Ϊ��BD��
��3����CO�����ж����壬Ҫ����β�����������Դӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����ȱ��β������װ�ã�
�ʴ�Ϊ��ȱ��β������װ�ã�
�ڿ�ȼ��������������ϴﵽһ���̶�ʱ��������ᷢ����ը�������ȵ�ȼ�ֽⷴӦ���ľƾ��ƣ��ž�װ���еĿ��������ȵ�ȼA���ľƾ��ƣ�
�ʴ�Ϊ��A��
��ʵ������й۲쵽B�а�ɫ��ˮCuSO4�����ɫ��C�г���ʯ��ˮ����ǣ�E�к�ɫ������ɫ�����֤���������������ȷֽ�����������H2O��CO��CO2��
�ʴ�Ϊ��C�г���ʯ��ˮ����ǣ�E�к�ɫ��ĩ���ɫ��
�ܲ������������е���Ԫ������Ϊ��3.6g��$\frac{56}{180}$=1.12g���������������е���Ԫ����ȫת�����������У�
����������Ԫ�ص�����Ϊ��1.60g-1.12g=0.48g��
��Ԫ�غ���Ԫ�ص�������Ϊ��1.12g��0.48g=7��3��
������������Ļ�ѧʽΪFexOy��
����56x��16y=7��3��
x��y=2��3��
��������������Ļ�ѧʽΪFe2O3��
�������������ֽ����ɵIJ���ΪFe2O3��H2O��CO��CO2���䷴Ӧ����ʽΪ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe2O3+3CO��+CO2��+2H2O��
�ʴ�Ϊ��Fe2O3��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe2O3+3CO��+CO2��+2H2O��
���� ���⿼�����ε�ˮ�⡢��Һ�е���غ�������غ��Ӧ�á��ܶȻ�������Ӧ�á�����ʵ�鷽����Ƶȣ���Ŀ�漰��֪ʶ��϶࣬�ۺ��Խ�ǿ�������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������ʵ��̽��������

 �绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ����ͼ��ʾ��NH3������Ϊ���������ʣ�����˵��������ǣ�������
�绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ����ͼ��ʾ��NH3������Ϊ���������ʣ�����˵��������ǣ�������| A�� | ��Һ��OH-��缫a�ƶ� | |
| B�� | ��Ӧ���ĵ�NH3��O2�����ʵ���֮��Ϊ4��5 | |
| C�� | O2�ڵ缫b�Ϸ�����ԭ��Ӧ | |
| D�� | �����ĵ缫��ӦʽΪ��2NH3-6e-+6OH-=N2��+6H2O |
| A�� | �ڴ������Ƕп�鱣��������пΪԭ��صĸ��� | |
| B�� | MgO���۵����NaCl������ΪMgO�ľ����ܴ���NaCl | |
| C�� | 1molFeCl3��ȫˮ�⽫����6.02��1023���������� | |
| D�� | ˮ�����ӻ�����Ksp�����¶ȵ����߶�����˵��ˮ�ĵ��������ȹ��� |
| A�� | pH��ͬ�Ģ�CH3COONa����NaHCO3����C6H5ONa������Һ�е�c��Na+�����ۣ��ڣ��� | |
| B�� | �����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��Na2CO3��Һ��NaHCO3��Һ��������������Һ�У�2c��OH-��-2c��H+��=3c��H2CO3��+c��HCO3-��-c��CO32-�� | |
| C�� | ��Ũ�ȡ��������Na2CO3��NaHCO3��ϣ�$\frac{c��HC{O}_{3}^{-}��}{c��{H}_{2}C{O}_{3}��}$��$\frac{c��C{O}_{3}^{2-}��}{c��HC{O}_{3}^{-}��}$ | |
| D�� | ������AgCl�ֱ���룺��5mLˮ ��10mL 0.2mol/L MgCl2 ��20mL 0.3mol/L���� ���ܽ�������c��Ag+�����٣��ڣ��� |
| A�� | ����Ӧ��H��0 | |
| B�� | n��NH3��/n��SO2����a��b��c | |
| C�� | ��ͬ�����£���������Խ��SO2��ƽ��ת����Խ�� | |
| D�� | ��ʱ����ϵ�г�ȥˮ��ƽ�ⳣ������ |
 ��1�����з�Ӧ�������ȷ�Ӧ����DE
��1�����з�Ӧ�������ȷ�Ӧ����DE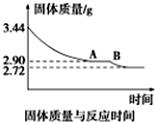 ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϣ�������ѧ֪ʶ������������⣺
ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϣ�������ѧ֪ʶ������������⣺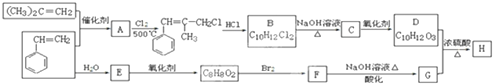
 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$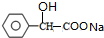 +NaBr+H2O��
+NaBr+H2O��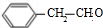 ��
��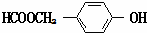 ��д�ṹ��ʽ����
��д�ṹ��ʽ���� ��֪A��B��C��D��E��F����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A��һ�ֵ�������Ȼ��Ӳ���������ʣ�B�ǵؿ��к�������Ԫ�أ�C�ĵ����ܴ�B��һ���⻯�����û���B��һ�ֵ��ʣ�D����������еij���Ԫ�أ�D���������ﳣ��������A��B�γɵ�һ�ֻ����F��ԭ��������E��1��F�Ǣ���Ԫ����ԭ��������С��һ��Ԫ�أ�
��֪A��B��C��D��E��F����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A��һ�ֵ�������Ȼ��Ӳ���������ʣ�B�ǵؿ��к�������Ԫ�أ�C�ĵ����ܴ�B��һ���⻯�����û���B��һ�ֵ��ʣ�D����������еij���Ԫ�أ�D���������ﳣ��������A��B�γɵ�һ�ֻ����F��ԭ��������E��1��F�Ǣ���Ԫ����ԭ��������С��һ��Ԫ�أ� ��
��