��Ŀ����
11�����������ֿ����Ի�������ɵĻ����Һ����Ũ�Ⱦ�Ϊ0.1mol•L-1�����������ӿ�����Fe2+��Al3+��Fe3+��H+�������ӿ�����NO3-��CO32-��I-��Cl-��������Һ�м��������ᣬ����Һ��ɻ�ɫ�������������ɣ�������ˮ����������������ӣ�������˵��һ����ȷ���ǣ���������| A�� | ����Һ�м���������ʱ�����ɵ�������ܺ���CO2 | |
| B�� | ԭ��Һ��һ������NO3-��Fe2+��һ������Fe3+��H+��CO32- | |
| C�� | ԭ��Һ��һ�����е�����ΪNO3-�����ܺ���Fe2+��I- | |
| D�� | ��ԭ��Һ����μ���NaOH��Һ���������������Ӻ���� |
���� ������Һ�м��������ᣬ����Һ��ɻ�ɫ��˵��ԭ��Һ�в�����H+����Һ��ɻ�ɫ˵��ԭ��Һ�в�����Fe3+�����������ɣ�������CO32-����H+�������ɶ�����̼������Һ�����ܳ��ֱ��ɫ��������Һ���������£��������ӻ�������������ӱ��ֳ�ǿ�������ԣ�����������������������������ͬʱ��������NO�����������ӻ�������������ӱ��ֳ�ǿ�������ԣ��������������ɵⵥ�ʣ����ֻ�ɫͬʱ��������NO����ԭ��Һһ������Fe2+��NO3-��һ������CO32-����ԭ��Һһ������I-��NO3-��������Fe2+����������ֻ����Al3+��Ҳһ������CO32-���ݴ˷������
��� �⣺A����ԭ��Һһ������Fe2+��NO3-��һ������CO32-����ԭ��Һһ������I-��NO3-��������Fe2+����������ֻ����Al3+��Ҳһ������CO32-����������Һ�м���������ʱ�����ɵ����岻���ܺ���CO2����A����
B���������ӻ�������������ӱ��ֳ�ǿ�������ԣ��������������ɵⵥ�ʣ����ֻ�ɫͬʱ��������NO��ԭ��Һ��һ������NO3-��һ������Fe3+��H+��CO32-����һ������Fe2+����B����
C���������ӻ�������������ӱ��ֳ�ǿ�������ԣ�����������������������������ͬʱ��������NO��4Fe2++NO3-+4H+=4Fe3++NO��+2H2O�����������ӻ�������������ӱ��ֳ�ǿ�������ԣ��������������ɵⵥ�ʣ����ֻ�ɫͬʱ��������NO��4I-+2NO3-+6H+=2I2��+2NO��+3H2O����C��ȷ��
D��������Al3+���ܴ��ڣ���ԭ��Һһ������Fe2+��ԭ��Һ����μ���NaOH��Һ����������һ�����ӵ������٣���D����
��ѡC��
���� ���⿼�����ӹ��桢������ƶϣ���Ŀ�Ѷ��еȣ�ע������ʵ������Լ���Ӧ�����ƶϣ���������Ԫ�ػ���������ʣ�

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�| �ṹ��ʽ | �е�/�� | �ṹ��ʽ | �е�/�� |
| CH3CH3 | -88.6 | CH2=CH2 | -103.7 |
| CH3CH2CH3 | -42.2 | CH2=CHCH3 | -47.4 |
| CH3CH2CH2CH3 | -0.5 | CH3CH2CH=CH2 | -6.3 |
 | -11.7 | 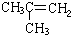 | -6.9 |
| CH3CH2CH2CH2CH3 | 36.1 | CH3CH2CH2CH=CH2 | 30 |
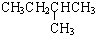 | 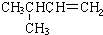 | 20.1 |
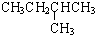 ��1.013��105Pa��25��ʱ��״̬����̬������̬��Һ̬���̬����
��1.013��105Pa��25��ʱ��״̬����̬������̬��Һ̬���̬������2��ʯ���ѽ���һ�����ӵĹ��̣������Ϊ�������磺
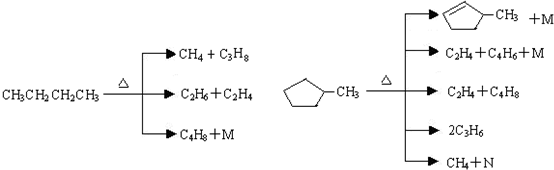
�����������ͻ������ѽⷴӦ�У�����M�Ļ�ѧʽΪH2��N�Ļ�ѧʽΪC5H8��
�������
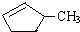 ��˵����ȷ����A��
��˵����ȷ����A��A��������ˮ����
 ��
��
B����������
 ��
��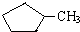 ��ȫȼ��ʱ�ĺ�������ͬ
��ȫȼ��ʱ�ĺ�������ͬC��
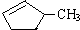 ����ˮ������Ȼ���
����ˮ������Ȼ�����3����ҵ���ѽⷴӦ�IJ��ﶼ����̬С����������ϩ���Ļ�����������һ����ķ����ǽ����¶ȣ�
| A�� | ��ҵ�Ͻ����Ṥҵβ��SO2����ͨ�백ˮ�У�SO2+OH-�THSO3- | |
| B�� | ��NaAlO2��Һ��ͨ�����CO2��AlO2-+CO2+2H2O�TAl��OH��3��+HCO3- | |
| C�� | �ö��Ե缫���KOH��Һʱ�����缫��Ӧ��2H2O-4e-�TO2��+4H+ | |
| D�� | NaHSO4��Һ��Ba��OH��2��Һ��ַ�Ӧ����Һ�����ԣ�Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O |
| A�� | C3H8O | B�� | C2H6O | C�� | C2H4O2 | D�� | C4H10 |
 pH=2��A��B��������Һ��lmL���ֱ��ˮϡ�͵�1000mL������Һ��pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������
pH=2��A��B��������Һ��lmL���ֱ��ˮϡ�͵�1000mL������Һ��pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | A��B��ҺŨ��һ����� | B�� | ϡ�ͺ�A��Һ���Ա�B��Һǿ | ||
| C�� | a=5ʱ��A��ǿ�ᣬB������ | D�� | ��A��B�������ᣬ��2��a=5 |

 ��ͼ��ʵ����������������װ�ã��ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ�ϣ�Ȼ����������Թܣ�ʹ֮��Ͼ��ȣ�
��ͼ��ʵ����������������װ�ã��ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ�ϣ�Ȼ����������Թܣ�ʹ֮��Ͼ��ȣ�