��Ŀ����
1��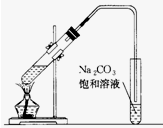 ��ͼ��ʵ����������������װ�ã��ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ�ϣ�Ȼ����������Թܣ�ʹ֮��Ͼ��ȣ�
��ͼ��ʵ����������������װ�ã��ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ�ϣ�Ȼ����������Թܣ�ʹ֮��Ͼ��ȣ���1��װ����ͨ�����ĵ���Ҫ���ڱ���Na2CO3��Һ��Һ���Ϸ������ܲ�����Һ�У�Ŀ���Ƿ�ֹNa2CO3��Һ�ĵ���
��2��Ũ����������Ǣٴ���������ˮ��
��3������Na2CO3��Һ���������ܽ��Ҵ����к�������������������ܽ�ȣ����ڷֲ�������
��4��ʵ�����ɵ��������������ܶȱ�ˮС�����С�������й�����ζ��
��5����ʵ�����¶ȹ��ߣ�ʹ��Ӧ�¶ȴﵽ140������ʱ������Ӧ����Ҫ�л����������ѣ� CH3CH2OCH2CH3 ����
���� ��1����ֹ���ڼ��Ȳ��������Na2CO3��Һ���������ȷ�Ӧ����Թ��У������Թ����ѣ�
��2���������Ҵ���Ũ�������������������������������������ڷ�ӦΪ���淴Ӧ��ͬʱŨ������ˮ������ƽ�����������������ķ����ƶ���
��3��ʵ�������ñ���̼������Һ��ȴ����������ԭ��ȥ������Ҵ��������������������ܽ�ȣ�����ˮ���ܶȣ�ʹ������ˮ�棬���ֲ����������ڷ��룻
��4���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������������ܶȱ�ˮС���ͼ�������ζ��
��5���Ҵ���Ũ���������¼��ȵ���140�淢�����Ӽ���ˮ�������ѣ�
��� �⣺��1�����Ȳ����������Na2CO3��Һ���������ȷ�Ӧ����Թ��У������Թ����ѣ�
�ʴ�Ϊ����ֹNa2CO3��Һ�ĵ�����
��2���������Ҵ���Ũ�������������������������������������ڷ�ӦΪ���淴Ӧ��ͬʱŨ������ˮ������ƽ�����������������ķ����ƶ���
�ʴ�Ϊ����������ˮ����
��3���Ʊ���������ʱ���ñ���̼������Һ����������������Ҫ���������������������ڱ���̼���ƣ��Ҵ���ˮ���ܣ������ܱ�̼�������գ����ڳ�ȥ���ʣ�
�ʴ�Ϊ���ܽ��Ҵ����к�������������������ܽ�ȣ����ڷֲ�������
��4���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��CH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O�����ɵ������������ܶ�С��ˮ���ܶȣ��й�����ζ��
�ʴ�Ϊ��С�����㣻
��5���Ҵ���140�棬��Ũ���������ˮ�������ѣ��ṹ��ʽΪ��CH3CH2OCH2CH3��
�ʴ�Ϊ������ CH3CH2OCH2CH3��
���� ���⿼�������������ķ�Ӧԭ�����Ʊ���������Ŀ�Ѷ��еȣ�ע���������������ķ�Ӧԭ����ʵ�����Ʒ�����ȷŨ���ᡢ����̼������Һ�����ü���ȡ��������ȷ����Ϊ�����Ĺؼ���

| A�� | ����Һ�м���������ʱ�����ɵ�������ܺ���CO2 | |
| B�� | ԭ��Һ��һ������NO3-��Fe2+��һ������Fe3+��H+��CO32- | |
| C�� | ԭ��Һ��һ�����е�����ΪNO3-�����ܺ���Fe2+��I- | |
| D�� | ��ԭ��Һ����μ���NaOH��Һ���������������Ӻ���� |
| A�� | 1��2-���������1��1-���ȱ��� | B�� | 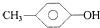 �� ��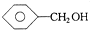 | ||
| C�� | CH3CH2CH2COOH��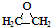 | D�� | ��Ȳ��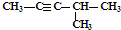 |
| A�� | ��ѧ��Ӧ�����ʱ仯��ʵ���Ǿɻ�ѧ���Ķ��Ѻ��»�ѧ�����γ� | |
| B�� | ���ӻ�������һ�����н���Ԫ�� | |
| C�� | ��ѧ����������ԭ�Ӽ�ǿ�ҵ������ | |
| D�� | ���ۻ������и�ԭ�Ӷ�һ�����������8�����ȶ��ṹ |
| A�� | �����CuO��NO��SO2��H2O | |
| B�� | �NaOH��KOH��Ba��OH��2��Na2CO3 | |
| C�� | ���������Na2O��CaO��Al2O3��Na2O2 | |
| D�� | ����ʣ�KNO3��Cl2��HCl��BaSO4 |
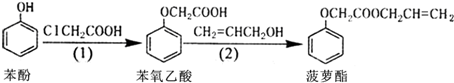
��������������ǣ�������
| A�� | ���ӺͲ���������������KMnO4��Һ������Ӧ | |
| B�� | ���ӡ���������Ͳ�����������NaOH��Һ������Ӧ | |
| C�� | ���裨1�����ɱ������ɱ�������ķ�Ӧ����ȡ����Ӧ | |
| D�� | ���裨2�������в�����ϩ������CH2=CHCH2OH��������ˮ���� |
| A�� | 2mol/LH2SO4��Һ | B�� | 2mol/LNaOH��Һ | ||
| C�� | 2mol/LMgSO4��Һ | D�� | �����ܽ����������Ӵ�����ɣ� |
| A�� | ����������Һ�����ᷴӦ Ba2++OH-+H++SO42-=BaSO4��+H2O | |
| B�� | �����ʯ��ˮ��ϡ���ᷴӦ Ca��OH��2+2H+=Ca2++2H2O | |
| C�� | ͭƬ������������Һ�� Cu+Ag+=Cu2++Ag | |
| D�� | ̼�ᱵ����ϡ������ BaCO3+2H+=Ba2++H2O+CO2�� |
 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ36��Ԫ�أ����ǵ�ԭ��������������Bԭ�ӻ�̬ʱPԭ�ӹ������3��δ�ɶԵ��ӣ����������������۴�����Ϊ2��C�ļ۲�����Ų�ʽΪns2npn+2�����⻯����ͬ��Ԫ�����γɵ��⻯���зе��������AC2Ϊ�Ǽ��Է��ӣ�DԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ�E��Ԫ�����ڱ��������ڵ�9��Ԫ�أ���ش�
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ36��Ԫ�أ����ǵ�ԭ��������������Bԭ�ӻ�̬ʱPԭ�ӹ������3��δ�ɶԵ��ӣ����������������۴�����Ϊ2��C�ļ۲�����Ų�ʽΪns2npn+2�����⻯����ͬ��Ԫ�����γɵ��⻯���зе��������AC2Ϊ�Ǽ��Է��ӣ�DԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ�E��Ԫ�����ڱ��������ڵ�9��Ԫ�أ���ش�