��Ŀ����
3����֪����298K��100kPaʱ����C��s��ʯī��+O2��g���TCO2��g����H1=-400kJ•mol-1��
��2H2��g��+O2��g���T2H2O��l����H2=-570kJ•mol-1��
��2C2H2��g��+5O2��g���T4CO2��g��+2H2O��l����H3=-2600kJ•mol-1��
��1��д��298Kʱ��C��s��ʯī����H2��g������1mol C2H2��g����Ӧ���Ȼ�ѧ����ʽC��s��ʯī��+2H2��g��=C2H2��g����H=+215kJ•mol-1��
��2������һ��������Ȳ��������������ȫȼ�գ��ų�����650kJ������Ӧ��Ķ�����̼���建��ͨ�뵽����0.5mol Ca ��OH��2�ij���ʯ��ˮ�г�ַ�Ӧ��
����Ӧ������ʷ�Ϊa��b��c���ȷݣ��ֱ��������ʵ�飬�ش���Ӧ���⣺
����a�м�������������������Һ��д����Ӧ�����ӷ���ʽCa2++HCO3-+OH-=CaCO3��+H2O��
����c�м���������FeCl3��Һ���۲쵽��ʵ���������������壬ͬʱ���ɺ��ɫ������
���� 1��������֪��Ӧ��˹���ɼ���õ������Ȼ�ѧ����ʽ��
��2����������Ȳ������ȼ������ɵĶ�����̼���ʵ�������������������ʵ���������Ӧ����ɷ�д��������������Һ��Ӧ�����ӷ���ʽ��
�����ݼ�������õ��ķ�Ӧ����ɷַ�����д���ӷ���ʽ��
��� �⣺��1����C��s��ʯī��+O2��g��=CO2��g����H1=-400kJ•mol-1��
��2H2��g��+O2��g��=2H2O��l����H2=-570kJ•mol-1��
��2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H3=-2600kJ•mol-1��
���ݸ�˹���ɢ١�4+��-�۵õ���4 C��s��ʯī��+2H2��g��=2C2H2��g����H=+430kJ•mol-1��
д��298Kʱ��C��s��ʯī����H2��g������1 mol C2H2��g����Ӧ���Ȼ�ѧ����ʽ��2 C��s��ʯī��+H2��g��=C2H2��g����H=+215kJ•mol-1��
�ʴ�Ϊ��C��s��ʯī��+2H2��g��=C2H2��g����H=+215kJ•mol-1��
��2��һ��������Ȳ��������������ȫȼ�գ��ų�����650kJ������2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H3=-2600kJ•mol-1������õ����ɶ�����̼���ʵ���Ϊ$\frac{650KJ��4}{2600KJ}$=1mol������Ӧ��Ķ�����̼���建��ͨ�뵽����0.5mol Ca ��OH��2�ij���ʯ��ˮ�г�ַ�Ӧ�����ݶ�����̼���������Ʒ�Ӧ������ϵ��������жϿ�֪����̼����ƣ���Ӧ�Ļ�ѧ����ʽΪ��2CO2+Ca��OH��2=Ca��HCO3��2��
����a�м�������������������Һ��д����Ӧ�����ӷ���ʽ��Ca2++HCO3-+OH-=CaCO3��+H2O��
�ʴ�Ϊ��Ca2++HCO3-+OH-=CaCO3��+H2O��
����c�м���������FeCl3��Һ�������Ӻ�̼��������ӷ���˫ˮ�����ɶ�����̼����������������������Ӧ�����ӷ���ʽΪFe3++3HCO3-=Fe��OH��3��+3CO2��������Ϊ�������壬ͬʱ���ɺ��ɫ������
�ʴ�Ϊ���������壬ͬʱ���ɺ��ɫ������
���� ���⿼�����Ȼ�ѧ����ʽ��˹���ɵļ���Ӧ�ã���ѧ��Ӧ����Ķ��������жϣ����ӷ���ʽ��д������ע������˫ˮ�ⷴӦ�������жϣ���Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����Һ�м���������ʱ�����ɵ�������ܺ���CO2 | |
| B�� | ԭ��Һ��һ������NO3-��Fe2+��һ������Fe3+��H+��CO32- | |
| C�� | ԭ��Һ��һ�����е�����ΪNO3-�����ܺ���Fe2+��I- | |
| D�� | ��ԭ��Һ����μ���NaOH��Һ���������������Ӻ���� |
| A�� | 14C��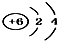 | B�� | 16O��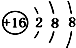 | C�� | Li+��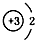 | D�� | H-��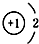 |
| A�� | �ڻ�ѧ��Ӧ��һ�������������ɣ�ͬʱҲһ���������ı仯 | |
| B�� | �ڻ�ѧ��Ӧ��һ�������������ɣ�����һ���������ı仯 | |
| C�� | �ڻ�ѧ��Ӧ�в�һ���л�ѧ���ı仯 | |
| D�� | �ڻ�ѧ��Ӧ��һ���л�ѧ���ı仯������һ���������ı仯 |
| A�� | �߷��ӷ���ĤӦ����ʳƷ��ҵ�У�������Ũ����Ȼ��֭������Ʒ�ӹ�������ҵ�� | |
| B�� | ���ϲ���һ������һ�ֲ��������壬��һ�ֲ�����Ϊ��ǿ�� | |
| C�� | �ϳɸ߷��Ӳ����Ƴɵ��˹�����һ�㶼�ܵ�������ų⣬���Դﵽ�������ݵij̶� | |
| D�� | ����������Ӧ���ڵ��ӹ�ҵ��һ�������л��߷��Ӳ��� |
| A�� | 1��2-���������1��1-���ȱ��� | B�� | 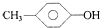 �� ��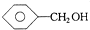 | ||
| C�� | CH3CH2CH2COOH��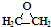 | D�� | ��Ȳ��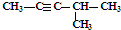 |
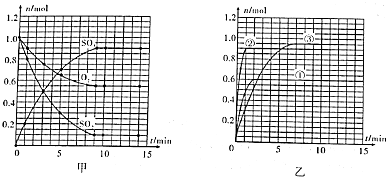
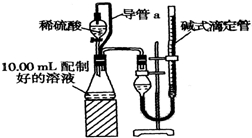 ʵ���Ҳⶨ̼���ƺ�̼�����ƻ������̼���Ƶ���������W��Na2CO3������ȡ�˻����5.0g������ˮ�У����250mL��Һ
ʵ���Ҳⶨ̼���ƺ�̼�����ƻ������̼���Ƶ���������W��Na2CO3������ȡ�˻����5.0g������ˮ�У����250mL��Һ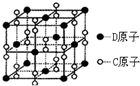 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ36��Ԫ�أ����ǵ�ԭ��������������Bԭ�ӻ�̬ʱPԭ�ӹ������3��δ�ɶԵ��ӣ����������������۴�����Ϊ2��C�ļ۲�����Ų�ʽΪns2npn+2�����⻯����ͬ��Ԫ�����γɵ��⻯���зе��������AC2Ϊ�Ǽ��Է��ӣ�DԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ�E��Ԫ�����ڱ��������ڵ�9��Ԫ�أ���ش�
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ36��Ԫ�أ����ǵ�ԭ��������������Bԭ�ӻ�̬ʱPԭ�ӹ������3��δ�ɶԵ��ӣ����������������۴�����Ϊ2��C�ļ۲�����Ų�ʽΪns2npn+2�����⻯����ͬ��Ԫ�����γɵ��⻯���зе��������AC2Ϊ�Ǽ��Է��ӣ�DԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ�E��Ԫ�����ڱ��������ڵ�9��Ԫ�أ���ش�