��Ŀ����
7����ҵ����ȡ����淋�����ͼ��ͼ����ش��������⣺
��1����������ҵ������������У�B�豸������������¯�����з�����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��
��2���ںϳ��������������ͨ�������Ŀ���ǿ�ʹNOѭ�����ã�ȫ��ת����HNO3��
��3����������Ĺ����г������һЩ��������������������ַ���������
��Һ���շ���NO+NO2+2NaOH�T2NaNO2+H2O
NH3��ԭ����8NH3+6NO2$\frac{\underline{\;����\;}}{\;}$7N2+12H2O
����ƽ������Ӧ����ʽ��
���������ַ����У�������ɫ��ѧ����NH3��ԭ����
��4��ij���ʳ���NH3�Ʊ�NH4NO3����֪����NH3��NO�IJ�����96%��NO��HNO3�IJ�����92%������HNO3������ȥ��NH3������ռ�ܺ�NH3������������������ģ���53.1%��
���� ��1��BΪ����¯��������¯�а�������������������һ��������ˮ��
��2��ͨ������������������ȷ��NO��ȫת�������
��3���ٸ��ݻ��ϼ����������ƽ�÷�Ӧ�ķ���ʽ��
�ھݷ�Ӧ��ѧ����ʽ��Ӧ�������������ʺ;���Ч���������ɫ��ѧΪ����Ⱦ��ԭ�������ʸߣ�
��4������NH3��NO�IJ�����96%��NO��HNO3�IJ�����92%�����õ�ԭ���غ��������������������HNO3��NH3��Ӧ����NH4NO3�����㰱����������������HNO3����ȥ��NH3������ռ�ܺ�NH3�����İٷ�����
��� �⣺��1����������ҵ������������У�B�豸�ǽ��а��Ĵ�������Ӧ��������¯�н��У�������Ӧ�ķ���ʽΪ��4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��
�ʴ�Ϊ������¯��4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��
��2���ںϳ��������������ͨ�������գ�ȷ���г����������ʹNOѭ�����ã�ȫ��ת����HNO3��
�ʴ�Ϊ����ʹNOѭ�����ã�ȫ��ת����HNO3��
��3���ٰ���������NԪ�صĻ��ϼ�Ϊ-3�ۣ�����������NԪ�صĻ��ϼ�Ϊ+4�ۣ�������NԪ�ػ��ϼ�Ϊ0�ۣ��������ɵ��������ϼ�����3�ۣ������������ɵ��������ϼ۽���4�ۣ��ϼ۱仯����С������Ϊ12��������ϵ��4������������ϵ��Ϊ3�����ڵ���Ϊ˫ԭ�ӷ��ӣ�������ϵ��Ӧ��Ϊ8������������ϵ��Ϊ6��Ȼ����������غ㶨����ƽ����ƽ��ķ�ӦΪ��8NH3+6NO2$\frac{\underline{\;����\;}}{\;}$7N2+12H2O��
�ʴ�Ϊ��8��6��7��12��
����ɫ��ѧ��Ҫ��Ӧ�Ի�������Ⱦ��ԭ�������ʸߵ��ŵ㣬����NH3��ԭ��������ɫ��ѧ��
�ʴ�Ϊ��NH3��ԭ����
��4�����ݵ�ԭ���غ��֪��NH3��NO��HNO3��
��1mol�����ɵõ���������ʵ���Ϊ��1mol��96%��92%=0.8832mol��
����HNO3+NH3�TNH4NO3��֪��Ӧ���ĵİ��������ʵ���Ϊ��0.8832mol��
����������֮�ȵ������ʵ���֮�ȣ�
����HNO3����ȥ��NH3������ռ�ܺ�NH3�����İٷ���Ϊ��$\frac{1mol}{1mol+0.8832mol}$��100%��53.1%��
�ʴ�Ϊ��53.1��
���� ���⿼���˹�ҵ�Ʊ�ԭ��Ӧ�á����̷�����ʵ���Ʊ����ʵķ����жϼ��������ʵ�ת���������غ�ķ��������м��㣬��Ŀ�Ѷ��еȣ���ȷ��ϵʽ���ڻ�ѧ�����е�Ӧ�ã�ע�����չ�ҵ��ȡ����ķ�Ӧԭ����

| A�� | 0.1 mol•L-1Ba��OH��2��Һ�к���0.2nA��OH- | |
| B�� | 1mol Na������ˮ��Ӧ��ת��2nA������ | |
| C�� | ���³�ѹ�£�22.4L��ϩ����nA��CH2=CH2���� | |
| D�� | ���³�ѹ�£�46g NO2����2nA����ԭ�� |
��1��FԪ�ؼ۲�����Ų�ʽΪ3d64s4��
��2������B2A2������˵������ȷ���Ǣڢܣ�
��B2A2�е�����ԭ�Ӷ�����8�����ȶ��ṹ
��B2A2���ɼ��Լ��ͷǼ��Լ��γɵķǼ��Է���
��ÿ��B2A2�����ЦҼ��ͦм���Ŀ��Ϊ1��1
��B2A2�����е�A-B������s-sp�Ҽ�
��3��B��C��D����Ԫ�ص�һ�����ܰ��ɴ�С��˳������ΪN��O��C����Ԫ�ط��ű�ʾ����B��C��D����Ԫ������BD2��Ϊ�ȵ�����ķ���ʽΪN2O������Ԫ�ط��ű�ʾ��
��4��A2E��������ԭ�ӵ��ӻ�����Ϊsp3���Ƚ�A2D��A2E���ӵķе㣬���зе�ϸߵ�ԭ��ΪH2O����֮����������Ԫ��D���γ�����ͬ�������壬������ˮ���ܽ�ȸ������O3�������ʽ����
��5��F���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ����Ϣ���£�
| ��� | �ѻ���ʽ | �����ⳤ��cm�� |
| �� | ��������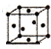 | a |
| �� | ��������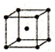 | b |
H2��g��+Cl2��g����2HCl��g��+Q1
H2��g��+Br2��g����2HBr��g��+Q2 ����������Ӧ�������ж���ȷ���ǣ�������
| A�� | Q1��Q2 | |
| B�� | �����������������ڷ�Ӧ�������� | |
| C�� | ����1mol HCl��g���ų�Q1���� | |
| D�� | �����ʵ���ʱ��Br2��g�����е���������Br2��l�� |
| A�� | ȡ��ȡҺ����������AgNO3��Һ�а�ɫ��������������ܺ���Cl- | |
| B�� | ȡ��ȡҺ����������Cu��ŨH2SO4���Թܿ��к���ɫ�������������ܺ���NO3- | |
| C�� | ȡ��ȡҺ���������������ữ��BaCl2��Һ���а�ɫ������������һ����SO42- | |
| D�� | �ýྻ�IJ�˿��պȡ��ȡҺ���ھƾ������������գ���ɫ�ʻ�ɫ����һ������Na+ |
| T/�� | 30 | 40 | 50 |
| NH3��ƽ��Ũ��/����10-6mol/L�� | 4.8 | 5.9 | 6.0 |
| A�� | �÷�Ӧ�ġ�H��0 | |
| B�� | �����¸÷�Ӧ�ġ�S��0 | |
| C�� | ��Ӧ��30�桢40��ʱ��ѧƽ�ⳣ���ֱ�ΪK1��K2����K1��K2 | |
| D�� | �ڳ��³�ѹ�£��˹��̵��Ƚ������� |
| A�� | ������������Z��R��Y | |
| B�� | ��̬�⻯����ȶ��ԣ�Y��Z | |
| C�� | R��X���������Ϊ���ӻ����� | |
| D�� | X��Y��������������Ӧ��ˮ���������Ӧ |
| A�� | NO�Ի�����Σ�������ƻ������㡢�γ�����ȷ��� | |
| B�� | NO������ijЩ��N���ʵĻ�ԭ����Ҳ������ijЩ���ͼ�N���ʵ��������� | |
| C�� | ʵ������ȡ��NO����������ˮ���������ſ������ռ� | |
| D�� | �������ڴ�������NO��������������ܣ�������Ѫ�ܡ��ٽ������� |
 CO2��
CO2�� MgCl2��
MgCl2�� Na2O2��
Na2O2�� ��
��