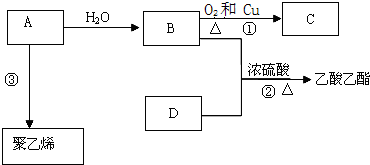
��Ŀ����
2����ҵ�ϳ���ˮ��������������Ӧ��ȡ������ʹҩ��˾ƥ�֣�����ˮ���ᣩ������Ӧԭ����
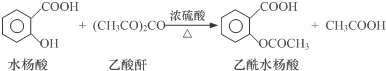
���������ʡ�
| �Լ� | �е㣨�棩 | �ܽ�� | ��ѧ���� |
| ˮ���� | 211 | ������ˮ����������ˮ | |
| ������ | 139 | ��ˮ���ֽ� | |
| ����ˮ���� | ����ˮ | ��̼���Ʒ�Ӧ����ˮ������ |
��1�������Ʊ�����125mL����ƿ�����μ���4gˮ���ᡢ10mL��������0.5mLŨ���ᣬ����ƿ��ˮ����ȫ���ܽ⣬��85�桫90�������£�����ˮԡ����5��10min��
�ټ���ˮ���ᡢ���������軺���μ�Ũ���ᣬ������ʻ��ͣ���ԭ����ˮ�������ڷ������ʣ��ᱻŨH2SO4������
�ڿ��Ʒ�Ӧ�¶�85�桫90���ԭ��ȱ�֤�нϸߵķ�Ӧ�����ּ��������ʵĻӷ���
��2����Ʒ�ᾧ��ȡ����ƿ������50mL����ˮ��ȴ����������ȫ�������ò���©�����ˣ���ϴ�Ӿ��壬��ɣ���Ҫ�������ϴ�Ӳ���©���еľ��壿����©���м����ˮ����û���о��壬�ٳ��ˣ��ظ�2��3�Σ�
��3����Ʒ�ᴿ�����ֲ�Ʒת����150mL�ձ��У����������������Լ�X�����Ͻ��������ٲ�������Ϊֹ����һ���ᴿ���ջ������ˮ����3.6g��
���Լ�XΪ����̼������Һ��
��ʵ��������ˮ����IJ���Ϊ69%����֪��ˮ���ᡢ����ˮ�������Է��������ֱ�Ϊ138��180����
��4�����ȼ��飺ȡ������Ʒ����ʢ��5mLˮ���Թ��У�����1��2��FeCl3��Һ����Һ��dz��ɫ������ܵ�ԭ���Dz�Ʒ����Ȼ���ܺ���ˮ���ᣮ
���� ��1����ˮ���Ậ�з��ǻ����ᱻŨH2SO4������
���¶�Ӱ�췴Ӧ���ʣ�����л����ӷ����з������
��2������©���м����ˮ����û���о��壬�ٳ��ˣ��ظ�2��3�Σ�
��3��ϴ�ӳ��˵õ��ֲ�Ʒ�к�������Ϊˮ���ἰ�������ᣬ�ñ���̼������Һ��ȥ���
����ˮ�����������������ˮ��������۲�������Ʒ����=��ʵ�ʲ��������۲�������100%��
��4��ȡ������Ʒ����ʢ��5mLˮ���Թ��У�����1��2��FeCl3��Һ����Һ��dz��ɫ��˵�����з������ʣ�
��� �⣺��1����ˮ���Ậ�з��ǻ������ڷ������ʣ��ᱻŨH2SO4�������軺���μ�Ũ���ᣬ�����������������ʻ��ͣ�
�ڿ��Ʒ�Ӧ�¶�85�桫90�棬�ȱ�֤�нϸߵķ�Ӧ�����ּ��������ʵĻӷ���
�ʴ�Ϊ��ˮ�������ڷ������ʣ��ᱻŨH2SO4�������ȱ�֤�нϸߵķ�Ӧ�����ּ��������ʵĻӷ���
��2��ϴ�Ӳ���©���еľ���ķ���Ϊ������©���м����ˮ����û���о��壬�ٳ��ˣ��ظ�2��3�Σ�
�ʴ�Ϊ������©���м����ˮ����û���о��壬�ٳ��ˣ��ظ�2��3�Σ�
��3��ϴ�ӳ��˵õ��ֲ�Ʒ�к�������Ϊˮ���ἰ�������ᣬ�ñ���̼������Һ��ȥ���
�ɷ���ʽ��֪������ˮ��������۲���Ϊ$\frac{4g}{138g/mol}$��180g/mol����Ʒ����=[3.6g��$\frac{4g}{138g/mol}$��180g/mol]��100%=69%��
�ʴ�Ϊ������̼������Һ��69%��
��4��ȡ������Ʒ����ʢ��5mLˮ���Թ��У�����1��2��FeCl3��Һ����Һ��dz��ɫ��˵�����з������ʣ���Ʒ����Ȼ���ܺ���ˮ���ᣬ
�ʴ�Ϊ����Ʒ����Ȼ���ܺ���ˮ���ᣮ
���� ���⿼���л����Ʊ�ʵ�鷽�����漰�Բ������������Ƶķ������ۡ�ϴ�ӡ����ʵķ����ᴿ�����ʼ���ȣ����ؿ���ѧ����֪ʶ��Ǩ�����á��������������������Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�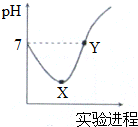 �����£���Cl2����ͨ��һ����������ˮ�������ͣ�Ȼ�������ñ�����ˮ����μ���0.1mol•L-1��NaOH��Һ������������pH�仯��ͼ��ʾ������������ȷ���ǣ�������
�����£���Cl2����ͨ��һ����������ˮ�������ͣ�Ȼ�������ñ�����ˮ����μ���0.1mol•L-1��NaOH��Һ������������pH�仯��ͼ��ʾ������������ȷ���ǣ�������| A�� | X��Y������ʾ��Һ�к�����������ǰ�߶� | |
| B�� | X��Y������ʾ��Һ��ˮ�ĵ���̶�ǰ�ߴ� | |
| C�� | Y����ʾ��Һ�д���c��Na+��=c��HClO��+2c��ClO-�� | |
| D�� | X����ʾ��Һ�з�����Ӧ�����ӷ���ʽΪCl2+H2O�T2 H++Cl-+ClO- |
| A�� | �����淴Ӧ���ʶ�����ƽ�ⲻ�ƶ� | |
| B�� | �����淴Ӧ���ʶ����䣬ƽ�ⲻ�ƶ� | |
| C�� | �����淴Ӧ���ʶ�����ƽ�������ƶ� | |
| D�� | �����淴Ӧ���ʶ�����ƽ�������ƶ� |
| A�� | �����ʯ��ˮ�м�������NaOH���壬��Һ�����ֻ��� | |
| B�� | ��Na2CO3��Һ��ͨ��CO2���壬һ���л��� | |
| C�� | ���������CuSO4��Һ�м���16gCuSO4�������¶Ȳ��䣬������������25g | |
| D�� | ����MnO2�뺬4mol HCl��Ũ���Ṳ�ȣ���ַ�Ӧ������Cl2�����ʵ���С��1mol |
ʪ���Ʊ�����Ҫ��Ӧ����Ϊ��2Fe��OH��3+3NaClO+4NaOH=2Na2FeO4+3NaCl+5H2O
�ɷ��Ʊ�����Ҫ��Ӧ����Ϊ��2FeSO4+4Na2O2=2Na2FeO4+2Na2SO4
�����йظ÷�Ӧ��˵��������ǣ�������
| A�� | ����Ӧ��Na2FeO4��Ϊ�������� | |
| B�� | Na2FeO4��ǿ�����ԣ�������ɱ�����仹ԭ����������ˮ������ | |
| C�� | �ɷ���ÿ����1mol Na2FeO4ת��3mol���� | |
| D�� | ���������£�NaClO�����Դ���Na2FeO4 |
| A�� | CH4O��C2H4O2 | B�� | C8H10��C4H10 | C�� | C2H4��C2H4O | D�� | C8H8��C4H8O3 |
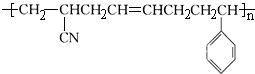 ��������˸߷��Ӳ�����ص�˵����ȷ���ǣ�������
��������˸߷��Ӳ�����ص�˵����ȷ���ǣ�������| A�� | �ϳɸø߷��Ӳ��ϵķ�Ӧ�����۷�Ӧ | |
| B�� | �ø߷��Ӳ����������ֵ���ۺ϶��ɵ� | |
| C�� | �ϳɸø߷��Ӳ��ϵIJ��ֵ��岻��ʹ��ˮ�����Ը��������Һ��ɫ | |
| D�� | �ø߷��Ӳ��������ͽṹ�߷��ӣ������ȹ��� |
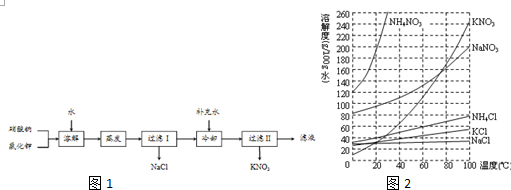
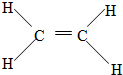 ��
��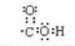 ��
��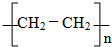 ��Ӧ���ͣ��Ӿ۷�Ӧ��
��Ӧ���ͣ��Ӿ۷�Ӧ��