��Ŀ����
7��3-��ͪ���������л��ϳ�����;�ܹ㣬�㷺����ҩ��ϳɣ�������ʳƷ�������������Է�������Ϊ130��������Ϊ��ɫҺ�壬�е�181�棬�����¶ȳ���95�����϶�ʱ�ͻ�ֽ⣻������ˮ�����Ҵ��������������л��Լ�������Ȼ��ܣ�ʵ���ҿ��������������ͽ�����Ϊԭ���Ʊ�������������Է�������Ϊ88��������Ϊ��ɫ�ӷ�Һ�壬����ˮ���е�77�森����Ӧԭ����
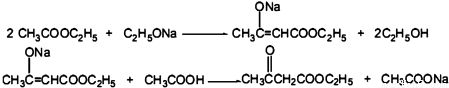
��ʵ��װ�á�

1�����ȷ�Ӧ����Ӧװ���м���32mL��28.5g��0.32mol������������������ˮ�Ҵ���1.6g��0.07mol����ϸ�Ľ����ƣ��Ȼ���1.5��3Сʱ��ֱ����������ʧ��
2�������������ȴ�����£�ж�������ܣ�����ƿ������ˮԡ�У���ҡ���»����ļ���32mL30%����ˮ��Һ��ʹ��ӦҺ�ֲ㣮�÷�Һ©����������㣮������5%̼������Һϴ�ӣ��л������������ƿ�У�������ˮ̼�����Һ����壮
3������δ��Ӧ����������������ӦҺ�ڳ�ѹ��������100�森Ȼ����ü�ѹ���õ���Ʒ2.0g��
�ش��������⣺
��1��д��ʵ�����Ʊ����������Ļ�ѧ����ʽCH3COOH+CH3CH2OH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����2����Ӧװ���мӸ������Ϊ�˷�ֹ�����е�ˮ�������뷴Ӧ��ϵ���Ա�֤��Ӧ��ϵ�������װ���������ܵ����ò���ͬ�����ͬ������ͬ��������ȴˮ��ˮ�ڷֱ�Ϊb��d����ͼ�е���ĸ����
��3����������У��μ�ϡ�����Ŀ�����к����ɵ����Σ�ʹ֮��ɲ���÷�Һ©����������������з�Һ��̼������Һϴ�ӵ�Ŀ���dz�ȥ���еĴ��ᣮ��̼��ص�Ŀ���Ǹ����Ʒ��
��4�����ü�ѹ�����ԭ����3-��ͪ�������е�ߣ��������¶ȳ���95�����϶�ʱ�ͻ�ֽ⣬������Ҫ��ѹ����
��5����ʵ�����õ���3-��ͪ������������B������ȷ�𰸱�ţ���
A��10% B��22% C��19% D��40%
���� ��1���������Ҵ���Ũ���ᡢ���������·���������Ӧ��������������ˮ��
��2�������к���ˮ���������װ���мӸ������Ϊ�˷�ʪ�����뷴Ӧ��ϵ���Ա�֤��Ӧ��ϵ���
��Ӧװ���������ܵ�����������������ͦ��ԭ�ϵ������ʣ���ѹװ���������ܵ���������ȴ���������������ڵõ���Ʒ��
Ϊ���������ʹ��ˮ�����������������ȴˮ��ȡ����ԭ����
��3�����ڲ����к������Σ���˲��ﴦ���μ�ϡ�����Ŀ�����к����ɵ����Σ�ʹ֮��ɲ��
���뻥������Һ�����Ϊ��Һ��
������̼������Һ�е��ܽ��С���μ�̼������Һ���Գ�ȥ���е����
̼�����ˮ����˼�̼��ص�Ŀ���Ǹ����Ʒ��
��4��3-��ͪ�������е�ߣ��������¶ȳ���95�����϶�ʱ�ͻ�ֽ⣬������Ҫ��ѹ����
��5�����ݷ�Ӧ���������֪�Ʋ��㣬������������ɲ�Ʒ�����ʵ�����0.07mol������m=nM����������3-��ͪ������������������������ʣ�
��� �⣺��1��ʵ������ȡ���������Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��2�������к���ˮ���������װ���мӸ������Ϊ�˷�ֹ�����е�ˮ�������뷴Ӧ��ϵ���Ա�֤��Ӧ��ϵ���
��Ӧװ���������ܵ�����������������ͦ��ԭ�ϵ������ʣ���ѹװ���������ܵ���������ȴ���������������ڵõ���Ʒ��Ϊ���������ʹ��ˮ��������������ȴˮ��ˮ�ڷֱ�Ϊb��d��
�ʴ�Ϊ����ֹ�����е�ˮ�������뷴Ӧ��ϵ���Ա�֤��Ӧ��ϵ�������ͬ��b��d��
��3�����ڲ����к������Σ���˲��ﴦ���μ�ϡ�����Ŀ�����к����ɵ����Σ�ʹ֮��ɲ����������˻����Ӳ�����ˮ�е��ܽ�ȣ�����ܵμӵĹ��ࣻ
�÷�Һ©�����������IJ����з�Һ��
������̼������Һ�е��ܽ��С����ȥ���еĴ��ᣬ��Ҫ�μ�̼������Һ��
̼�����ˮ����˼�̼��ص�Ŀ���Ǹ����Ʒ��
�ʴ�Ϊ���к����ɵ����Σ�ʹ֮��ɲ����Һ����ȥ���еĴ�������Ʒ��
��4��3-��ͪ�������е�ߣ��������¶ȳ���95�����϶�ʱ�ͻ�ֽ⣬������Ҫ��ѹ����
�ʴ�Ϊ��3-��ͪ�������е�ߣ��������¶ȳ���95�����϶�ʱ�ͻ�ֽ⣬������Ҫ��ѹ����
��5�����ݷ�Ӧ���������֪�Ʋ��㣬������������ɲ�Ʒ�����ʵ�����0.07mol����������0.07mol��130g/mol=9.1g�����Բ�����$\frac{2.0g}{9.1g}$��100%=22%����ѡ��B��
���� ���⿼���������Ʊ���������ơ���װ��������ķ������ۡ����ʵķ����ᴿ�����ʵļ��㣬�����ֿ�����ѧ���ķ���������������������ѧʵ���������Ѷ��еȣ�

| A�� | C3H8 | B�� | C3H7Cl | C�� | CH2Cl2 | D�� | C2H6O |
 ��1��T��ʱ��A������B���巴Ӧ����C���壬��Ӧ������A��B��CŨ�ȱ仯��ͼ��ʾ������ͼʾ��֪��A��B��Ӧ����C�Ļ�ѧ����ʽΪA��g��+3B��g��?2C��g����
��1��T��ʱ��A������B���巴Ӧ����C���壬��Ӧ������A��B��CŨ�ȱ仯��ͼ��ʾ������ͼʾ��֪��A��B��Ӧ����C�Ļ�ѧ����ʽΪA��g��+3B��g��?2C��g������2����80��ʱ����0.40mol ��N2O4�������2L �Ѿ���յĹ̶��ݻ����ܱ������У��������·�Ӧ��N2O4?2NO2����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
| ʱ��/s n/mol | 0 | 20 | 40 | 60 | 80 | 100 |
| n��N2O4�� | 0.40 | a | 0.20 | c | d | e |
| n��NO2�� | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
| A�� | ��Ӧ�й�����1.8 mol H2SO4 | B�� | �������SO2��H2�������Ϊ4��1 | ||
| C�� | ��Ӧ�й�����97.5 g Zn | D�� | ��Ӧ�й�ת��3 mol���� |
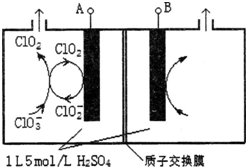 �о���Cl��N��S��Ԫ�صĻ�����Ծ���ˮ�ʡ�������Ⱦ����Ҫ���壮
�о���Cl��N��S��Ԫ�صĻ�����Ծ���ˮ�ʡ�������Ⱦ����Ҫ���壮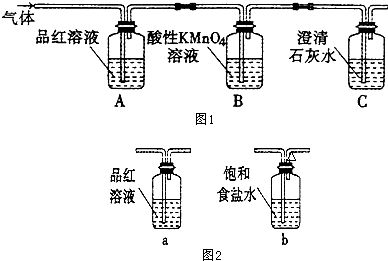
 ������A��B�ɺϳ�ӫ�⡰ħ�����в�������������֮һ��CPPO����
������A��B�ɺϳ�ӫ�⡰ħ�����в�������������֮һ��CPPO����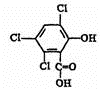 ��
�� ��
��