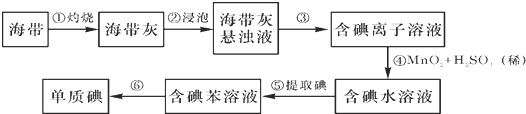
��Ŀ����
4�� ʵ����������0.2000mol•L -1Na2S2O3����Һ450mL�������ø���Һ��ijŨ�ȵ�NaClO��Һ���б궨��
ʵ����������0.2000mol•L -1Na2S2O3����Һ450mL�������ø���Һ��ijŨ�ȵ�NaClO��Һ���б궨����1������Na2S2O3���������Ʊ���Һ������ͼ��ʾ�������У�����Ҫ�õ���������AB������ĸ������ȱ�ٵIJ����������ձ��������������������ƣ���
��2�����ݼ���������ƽ��ȡNa2S2O3�����������15.8g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��=���������������=������ͬ��0.2000mol•L-1������δ����Һ��ȴ�Ͷ����ˣ���������ҺŨ�ȣ�0.2000mol•L-1��
��3���õζ����궨�ľ��巽������ȡ20.00mL NaClO��Һ����ƿ�У���������ϡ���������KI���壬��0.2000mol•L -1 Na2S2O3����Һ�ζ����յ㣨������Һ��ָʾ�������Ĵ�ƽ��ʵ��ⶨ��V��Na2S2O3���������£�
����֪��I2+2Na2S2O3�T2NaI+Na2S4O6��
| �ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| V��Na2S2O3��/mL | 21.90 | 18.80 | 22.10 | 22.00 |
��NaClO��Һ�����ʵ���Ũ����0.1060 mol•L -1��
���� ��1����Na2S2O3���������Ʊ���Һ������ҪԲ����ƿ����Һ©�����ܽ�Na2S2O3���壬����Ҫ�ձ�����������
��2��ʵ����û��450mL������ƿ��Ӧѡ��500mL������ƿ����ҪNa2S2O3���壬n=0.2000mol/L��0.5L=1.000mol��m=1.000mol��158g/mol=15.8g������ʱ������Ҫ������ƿ�м�������ˮ��������ƿ������ˮϴ�Ӻ�δ������������δ����Һ��ȴ�Ͷ����ˣ���ȴ����Һ�����С��������ҺŨ��ƫ��
��3����NaClO��Һ�м�������ϡ���������KI����ʱ����������ԭ��Ӧ�������ӷ���ʽΪClO-+2I-+2H+�TCl-+I2+H2O��
�������Ĵ�ƽ��ʵ��ⶨ��ƽ��V��Na2S2O3����
��NaClO��Һ��Ũ����x����ClO-+2I-+2H+�TCl-+I2+H2O��I2+2Na2S2O3�T2NaI+Na2S4O6
�ҵ���ϵʽ��NaClO��2Na2S4O6
1 2
20mL��c 0.2000mol/L��21.20mL
��֮�ã�c=0.1060mol/L��
��� �⣺��1����Na2S2O3���������Ʊ���Һ������ҪԲ����ƿ����Һ©�����ܽ�Na2S2O3���壬����Ҫ�ձ�����������
�ʴ�Ϊ��AB���ձ�����������
��2������0.2000mol•L -1Na2S2O3����Һ450mL��ʵ����û��450mL������ƿ��Ӧѡ��500mL������ƿ����ҪNa2S2O3���壬n=0.2000mol/L��0.5L=1.000mol��m=1.000mol��158g/mol=15.8g������ʱ������Ҫ������ƿ�м�������ˮ��������ƿ������ˮϴ�Ӻ�δ������������δ����Һ��ȴ�Ͷ����ˣ���ȴ����Һ�����С��������ҺŨ��ƫ��
�ʴ�Ϊ��15.8��=������
��3����NaClO��Һ�м�������ϡ���������KI����ʱ����������ԭ��Ӧ�������ӷ���ʽΪClO-+2I-+2H+�TCl-+I2+H2O��
�ʴ�Ϊ��ClO-+2I-+2H+�TCl-+I2+H2O��
���Ĵ�ƽ��ʵ��ⶨ��ƽ��V��Na2S2O3��=$\frac{21.90mL+18.80mL+22.10mL+22.00mL}{4}$=21.20mL��
��NaClO��Һ��Ũ����x����ClO-+2I-+2H+�TCl-+I2+H2O��I2+2Na2S2O3�T2NaI+Na2S4O6
�ҵ���ϵʽ��NaClO��2Na2S4O6
1 2
20mL��c 0.2000mol/L��21.20mL
��֮�ã�c=0.1060mol/L��
�ʴ�Ϊ��0.1060 mol•L -1��
���� ���⿼����Һ�����ơ�����������ϵʽ����ȣ���Ŀ�Ѷ��еȣ��ؼ�����ʵ����������������ԭ��Ӧ�жϷ��������ӷ�Ӧ���Ƕ�ѧ���ۺ������Ŀ��飮

| A�� | Ԫ�����ʵ������Ա仯��ָԭ�Ӱ뾶��Ԫ�ص���Ҫ���ϼۼ�ԭ�Ӻ�������Ų��������Ա仯 | |
| B�� | Ԫ�����ʵ������Ա仯������Ԫ��ԭ�ӽṹ�������Ա仯 | |
| C�� | ��Li��F��Na��Cl��Ԫ�ص�����ϼ۾����ִ�+1�ۡ�+7�۵ı仯 | |
| D�� | �������Ǿ��������������ϵ͵ĵ��Ӳ㣬��������K����L�㣬������M����N�� |
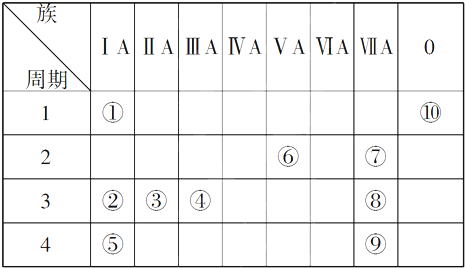
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� |
 ��
����2���ۢݢ�Ԫ��ԭ���γɼ����ӵ����Ӱ뾶�ɴ�С��˳����Cl-��F-��Al3+�������ӷ��ţ���
��3��ijԪ�ض��������ӵĺ�����10�����ӣ���Ԫ����þ����Ԫ�����ƣ���
��4���ܵĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��
 �����������ת�Ƶķ�������Ŀ����
�����������ת�Ƶķ�������Ŀ������5����Ԫ�صĵ��ʳ����µ���ɫ�ǻ���ɫ���õ���ʽ��ʾ��Ԫ�ص��⻯���γɹ���Ϊ
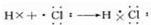 ��
����6����֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ����Ԫ�ص��������������Ƶ����ʣ�д��Ԫ�آٵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽBe��OH��2+2NaOH=Na2BeO2+2H2O��
| A�� | 1��4 | B�� | 2��3 | C�� | 1��7 | D�� | 2��5 |
| A�� | ZΪ0.3 mol•L-1 | B�� | X2Ϊ0.2 mol•L-1 | ||
| C�� | Y2Ϊ0.4 mol•L-1 | D�� | c��X2��+c��Y2��+c��Z��=0.55 mol•L-1 |
 �����������ոû��������ʻ�ɫ��
�����������ոû��������ʻ�ɫ��