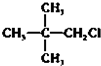
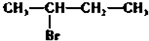��Ŀ����
15��Ԫ�����ڱ���Ԫ����������ѧϰ���о�������ʵ�����к���Ҫ�����ã��±��г��ˢ١������Ԫ�������ڱ��е�λ�ã�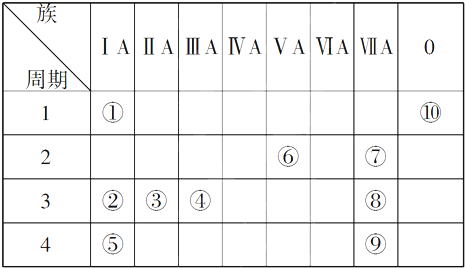
��ش𣺣���������Ӧ��Ԫ�ط��Żش����⣩
��1���ڱ�����д�١���Ԫ�ط��ţ�д��Ԫ�ط��Ţ�H����Mg����Cl��
��2�����л�ѧ��������õ���He����Ԫ�ط��ţ���
��3���ڢڡ��ۡ�������Ԫ�ص��������Ӧ��ˮ�����У�������ǿ�ļ���NaOH���ѧʽ����
��4��Ԫ�آ١��ڡ��ݰ�ԭ�Ӱ뾶�ɴ�С��˳������ΪK��Na��H����Ԫ�ط��ţ���ԭ��ͬ�������϶��µ��Ӳ��������࣬ԭ�Ӱ뾶������
��5��Ԫ�آ��⻯��Ļ�ѧʽ��NH3�����⻯������ˮ��������Һ��pH�������������������=����7�����⻯����ˮ������Ӧ�Ļ�ѧ����ʽΪNH3+H2O?NH3•H2O��
��6��Ԫ�آ���Ԫ�آ��γɵĻ�����Ļ�ѧʽ��NaCl������ʽ��
 �����������ոû��������ʻ�ɫ��
�����������ոû��������ʻ�ɫ����7��Al���ѧʽ���ĵ��ʼȿ��Ժ������ֿ��Ժ�����������Һ��Ӧ���䵥��������������Һ��Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
���� ��1����Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪNa����ΪMg����ΪAl����ΪK����ΪN����ΪF����ΪCl����ΪBr����ΪHe��
��2��ϡ������ԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�
��3��������Խǿ������������Ӧˮ����ļ���Խǿ��
��4��ͬ�������϶��µ��Ӳ������࣬ԭ�Ӱ뾶����
��5��Ԫ�آ��⻯��ΪNH3��������ˮ��Ӧ�õ�һˮ�ϰ���һˮ�ϰ������笠����������������ӣ���Һ�ʼ��ԣ�
��6��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪNaCl�����������������ӹ��ɣ����������ոû��������ʻ�ɫ��
��7��Al�ĵ��ʼȿ��Ժ������ֿ��Ժ�����������Һ��Ӧ���䵥��������������Һ��Ӧ����ƫ��������������
��� �⣺��1����Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪNa����ΪMg����ΪAl����ΪK����ΪN����ΪF����ΪCl����ΪBr����ΪHe���ʴ�Ϊ��H��Mg��Cl��
��2��ϡ������Heԭ�������Ϊ�ȶ��ṹ����ѧ��������ã��ʴ�Ϊ��He��
��3��ͬ����������ҽ����Լ���������Ԫ����Na�Ľ�������ǿ��������Խǿ������������Ӧˮ����ļ���Խǿ����NaOH�ļ�����ǿ���ʴ�Ϊ��NaOH��
��4��ͬ�������϶��µ��Ӳ��������࣬ԭ�Ӱ뾶������ԭ�Ӱ뾶��K��Na��H��
�ʴ�Ϊ��K��Na��H��ͬ�������϶��µ��Ӳ��������࣬ԭ�Ӱ뾶������
��5��Ԫ�آ��⻯��ΪNH3��������ˮ��Ӧ�õ�һˮ�ϰ���һˮ�ϰ������笠����������������ӣ���Һ�ʼ��ԣ�����ҺpH��7��������ˮ��Ӧ����ʽΪNH3+H2O?NH3•H2O��
�ʴ�Ϊ��NH3������NH3+H2O?NH3•H2O��
��6��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪNaCl�����������������ӹ��ɣ�����ʽΪ �����������ոû��������ʻ�ɫ��
�����������ոû��������ʻ�ɫ��
�ʴ�Ϊ��NaCl�� ���ƣ�
���ƣ�
��7��Al���ʼȿ��Ժ������ֿ��Ժ�����������Һ��Ӧ��������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��Al��2Al+2NaOH+2H2O=2NaAlO2+3H2����
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã��ѶȲ���ע���������Ԫ�����ڱ��ṹ���������ջ���֪ʶ��

 �屽����ɫ��״Һ�壬���б�����ζ��������ˮ���������л��ܼ���ʵ������ȡ�屽ʱ�Ƚ����ۺͱ����뷴Ӧ�����ڽ��������������屽������70-80��ˮԡ�з�Ӧ1h�����ô�Ʒ��ˮ��5%����������Һϴ�ӣ����÷ֲ㣬��������ˣ����ѹ����ȡ156-157����ֶ��ó�Ʒ����ȡװ����ͼ��ʾ��������������Ϣ�ش����⣮
�屽����ɫ��״Һ�壬���б�����ζ��������ˮ���������л��ܼ���ʵ������ȡ�屽ʱ�Ƚ����ۺͱ����뷴Ӧ�����ڽ��������������屽������70-80��ˮԡ�з�Ӧ1h�����ô�Ʒ��ˮ��5%����������Һϴ�ӣ����÷ֲ㣬��������ˣ����ѹ����ȡ156-157����ֶ��ó�Ʒ����ȡװ����ͼ��ʾ��������������Ϣ�ش����⣮| ���� | �� | �� | �屽 |
| �е� | 58.5�� | 80.1�� | 156.2�� |
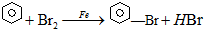 ��
����2������a������Ϊ������ƿ������c������������������������
��3����Ӧ����70-80��ˮԡ�ж���ֱ�Ӽ��ȵ�Ŀ����ʹ��Ӧ���Ⱦ��ȣ������ܼ��ٷ�Ӧ�Ļӷ�����ֹ���ɵ��屽�ӷ���
��4��ˮϴ����5%����������Һϴ�ӵ�Ŀ��Br2+2OH-=Br-+BrO-+H2O�������ӷ���ʽ��ʾ����
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
 ��
����2���õ���������ǿ����F �ؿ��к������Ľ���Ԫ����Al��
��3���õ���ʽ��ʾ������γɻ�����Ĺ���
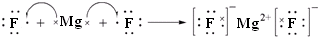 ��
����4����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH�������Ե�����������Al��OH��3��
��5���ȽϢڢۢܢ��γɵļ����Ӱ뾶�Ĵ�СCl-��F-��Na+��Mg2+��
��6��ijԪ��R����̬�⻯��ΪHxR����R�ڸ��⻯���е���������Ϊ94%��8.5g��HxR�����ڱ�״̬�µ������5.6L����HxR����Է�����Ϊ34��HxR�Ļ�ѧʽΪH2S��
| �� ���� | ��A | ��B | ��A | ��A | ��A | ��A | ��A | ||
| һ | �� | ||||||||
| �� | �� | �� | �� | �� | �� | ||||
| �� | �� | �� | �� | �� | |||||
| �� | ⑪ | ⑫ | ⑬ | ||||||
��2������Ԫ�آ��ԭ�ӽṹʾ��ͼ��
 ��
����3������Ԫ�آޢ���⻯����ȶ���˳��ΪHF��HCl��H2S����д��ѧʽ����ͬ����
��4������Ԫ�آ�͢�����������Ӧˮ��������ԣ�HClO4��H2SO4��
 ʵ����������0.2000mol•L -1Na2S2O3����Һ450mL�������ø���Һ��ijŨ�ȵ�NaClO��Һ���б궨��
ʵ����������0.2000mol•L -1Na2S2O3����Һ450mL�������ø���Һ��ijŨ�ȵ�NaClO��Һ���б궨����1������Na2S2O3���������Ʊ���Һ������ͼ��ʾ�������У�����Ҫ�õ���������AB������ĸ������ȱ�ٵIJ����������ձ��������������������ƣ���
��2�����ݼ���������ƽ��ȡNa2S2O3�����������15.8g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��=���������������=������ͬ��0.2000mol•L-1������δ����Һ��ȴ�Ͷ����ˣ���������ҺŨ�ȣ�0.2000mol•L-1��
��3���õζ����궨�ľ��巽������ȡ20.00mL NaClO��Һ����ƿ�У���������ϡ���������KI���壬��0.2000mol•L -1 Na2S2O3����Һ�ζ����յ㣨������Һ��ָʾ�������Ĵ�ƽ��ʵ��ⶨ��V��Na2S2O3���������£�
����֪��I2+2Na2S2O3�T2NaI+Na2S4O6��
| �ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| V��Na2S2O3��/mL | 21.90 | 18.80 | 22.10 | 22.00 |
��NaClO��Һ�����ʵ���Ũ����0.1060 mol•L -1��
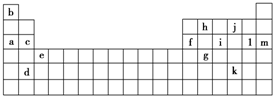
��1��������������Ԫ���е�һ�����ܣ�I1��������m������ĸ��ʾ����ͬ����c��f��I1��С��ϵ��c����f��
��2������Ԫ���У�ԭ����δ�ɶԵ�����������i��д����Ԫ�صĵ����Ų�ʽ��1s22s22p63s23p3��
��3�������±����ṩ�ĵ��������ݣ��ش��������⣮
| � | X | Y | |
| I1 | 519 | 502 | 580 |
| I2 | 7296 | 4570 | 1820 |
| I3 | 11799 | 6920 | 2750 |
| I4 | 9550 | 11600 |
��Y�����ڱ��еĢ�A���Ԫ�أ�