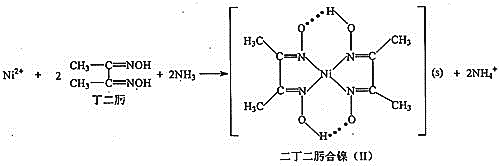
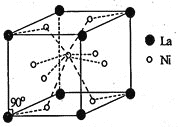
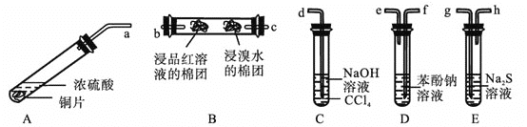
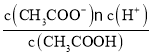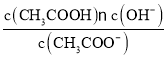题目内容
【题目】已知硫代硫酸钠在酸性条件下会发生反应:![]() ,下表中的两种溶液混合,出现浑浊的先后顺序是( )
,下表中的两种溶液混合,出现浑浊的先后顺序是( )
组号 | 两种溶液的温度 |
| 稀硫酸的体积、浓度 |
① | 15℃ | 10mL0.1mol/L | 50mL0.05mol/L |
② | 15℃ | 10mL0.05mol/L | 10mL0.1mol/L |
③ | 25℃ | 10mL0.05mol/L | 10mL0.1mol/L |
④ | 25℃ | 10mL0.1mol/L | 30mL0.07mol/L |
A.④①②③B.③④②①
C.④③②①D.④③①②
【答案】C
【解析】
根据表格中的浓度与体积的数据利用公式c混=![]() 可计算其混合液中各组分的浓度,结合温度越高,浓度越大,则反应速率越大,分析出现浑浊的先后顺序。
可计算其混合液中各组分的浓度,结合温度越高,浓度越大,则反应速率越大,分析出现浑浊的先后顺序。
根据表格中的浓度与体积的数据利用公式c混=![]() 可计算其混合液中各组分的浓度,如下表:
可计算其混合液中各组分的浓度,如下表:
组号 |
|
|
① |
|
|
② |
|
|
③ |
|
|
④ |
|
|
结合温度越高,浓度越大,则反应速率越大,可知④的反应速率最大,①的反应速率最小,因此出现浑浊的先后顺序为④③②①,故答案选C。

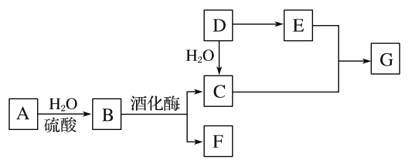
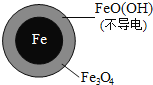
【题目】用零价铁![]() 去除水体中的硝酸盐
去除水体中的硝酸盐![]() 已成为环境修复研究的热点之一.
已成为环境修复研究的热点之一.
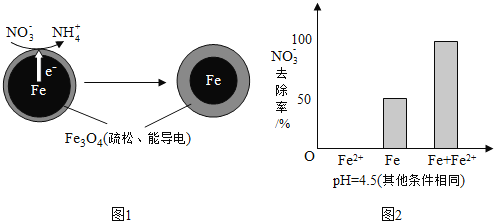
![]() 还原水体中
还原水体中![]() 的反应原理如图1所示.
的反应原理如图1所示.
①作负极的物质是______.
②正极的电极反应式是______.
![]() 将足量铁粉投入水体中,经24小时测定
将足量铁粉投入水体中,经24小时测定![]() 的去除率和pH,结果如下:
的去除率和pH,结果如下:
初始pH |
|
|
| 接近 |
|
24小时pH | 接近中性 | 接近中性 |
铁的最终物质形态 |
|
|
![]() 时,
时,![]() 的去除率低.其原因是______.
的去除率低.其原因是______.
![]() 实验发现:在初始
实验发现:在初始![]() 的水体中投入足量铁粉的同时,补充一定量的
的水体中投入足量铁粉的同时,补充一定量的![]() 可以明显提高
可以明显提高![]() 的去除率.对
的去除率.对![]() 的作用提出两种假设:
的作用提出两种假设:
Ⅰ![]() 直接还原
直接还原![]() ;
;
Ⅱ![]() 破坏
破坏![]() 氧化层.
氧化层.
①做对比实验,结果如图2所示,可得到的结论是______.
②同位素示踪法证实![]() 能与
能与![]() 反应生成
反应生成![]() 结合该反应的离子方程式,解释加入
结合该反应的离子方程式,解释加入![]() 提高
提高![]() 去除率的原因:______.
去除率的原因:______.
![]() 其他条件与
其他条件与![]() 相同,经1小时测定
相同,经1小时测定![]() 的去除率和pH,结果如表:
的去除率和pH,结果如表:
初始pH |
|
|
| 约 | 约 |
1小时pH | 接近中性 | 接近中性 |
与![]() 中数据对比,解释
中数据对比,解释![]() 中初始pH不同时,
中初始pH不同时,![]() 去除率和铁的最终物质形态不同的原因:______.
去除率和铁的最终物质形态不同的原因:______.