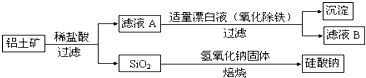
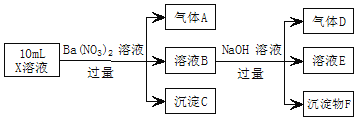
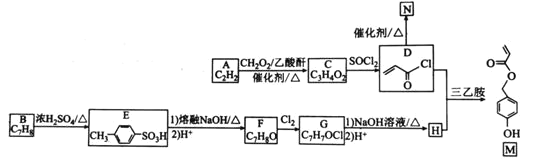
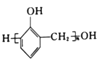
ЬтФПФкШн
ЁОЬтФПЁПНЙбЧСђЫсФЦ(Na2S2O5ЃКM=190 gЁЄmol-1)дкЪГЦЗМгЙЄжаГЃгУзїЗРИЏМСЁЂЦЏАзМСКЭЪшЫЩМСЁЃНЙбЧСђЫсФЦЮЊЛЦЩЋНсОЇЗлФЉЃЌ150ЁцЪБПЊЪМЗжНтЃЌдкЫЎШмвКЛђКЌгаНсОЇЫЎЪБИќвзБЛПеЦјбѕЛЏЁЃНЙбЧСђЫсФЦ(Na2S2O5)дкЪЕбщЪвПЩгУШчЭМзАжУжЦБИЁЃ
вбжЊЃК2NaHSO3![]() Na2S2O5+H2O S2O52-+2H+=2SO2Ёќ+H2O
Na2S2O5+H2O S2O52-+2H+=2SO2Ёќ+H2O
(1)ЪЕбщжаШчЙћSO2ЭЈШыЙ§ЖрЃЌЛсНЕЕЭNa2S2O5ЕФВњТЪЃЌЪдНтЪЭЦфдвђ__________ЁЃ
(2)ВтЖЈВњЦЗжаНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ГЃгУЪЃгрЕтСПЗЈ(дгжЪВЛгыЕтЗДгІ)ЁЃ
вбжЊЃКS2O52-+2I2+3H2O=2SO42-+4I-+6H+ЃЛ 2S2O32-+I2 =S4O62-+2I-
ГЦШЁ0.2000 gВњЦЗЗХШыЕтСПЦПжаЃЌМгШы40.00 mL 0.1000 molЁЄL-1ЕФБъзМЕтШмвКЃЌдкАЕДІЗХжУ5 minЃЌдйМгШыЪЪСПБљДзЫсМАеєСѓЫЎЁЃгУ0.2000 molЁЄL-1ЕФБъзМNa2S2O3ШмвКЕЮЖЈЪЃгрЕФЕтжСжеЕуЃЌжиИДВйзї3ДЮЃЌВтЕУЦНОљЯћКФБъзМNa2S2O3ШмвК20.00 mLЁЃдђВњЦЗжаНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ЮЊ__________________ЁЃ
(3)ЙигкБОЪЕбщЕФЫЕЗЈДэЮѓЕФЪЧ_________ЁЃ
A.BзАжУжаCCl4ЕФзїгУЪЧЗРжЙЕЙЮќЁЁ
B.ЕЮЖЈЪБЃЌNa2S2O3ШмвКгІИУгУМюЪНЕЮЖЈЙмЪЂЗХ
C.ЕЮЖЈжеЕуЕФЯжЯѓЪЧИеКУБфРЖЩЋ
D.ЕЮЖЈЪБМфЙ§ГЄЛсЕМжТВтЕУВњЦЗжаНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ЦЋИп
ЁОД№АИЁПЭЈШыЙ§ЖрЛсЕМжТЫсадЙ§ЧПЃЌЪЙНЙбЧСђЫсФЦгыH+ЗДгІЩњГЩSO2 95.00% CD
ЁОНтЮіЁП
(1)SO2гыH2OЗДгІВњЩњH2SO3ЃЌЕМжТШмвКЫсаддіЧПЃЌНсКЯбЧСђЫсФЦЕФаджЪЗжЮіЃЛ
(2)ЯШИљОнБъзМБъзМNa2S2O3ШмвКЯћКФЕФБъзМЕтШмвКЕФЮяжЪЕФСПЃЌШЛКѓгЩзмБъзМЕтШмвКЕФЮяжЪЕФСППЩЕУНЙбЧСђЫсФЦЯћКФЕФБъзМЕтШмвКЕФЮяжЪЕФСПЃЌРћгУЖўепЗДгІЙиЯЕМЦЫуГі0.2000 gВњЦЗжаКЌгаЕФНЙбЧСђЫсФЦЕФжЪСПЃЌНјЖјПЩЕУВњЦЗжаНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ЃЛ
(3)A.ИљОнSO2ЕФаджЪЗжЮіCCl4ЕФзїгУЃЛ
B.НсКЯбЮЕФЫЎНтЙцТЩХаЖЯЪЙгУЕФвЧЦїЃЛ
C.ИљОнЕтЫЎЕФбеЩЋБфЛЏХаЖЯЕЮЖЈжеЕуЃЛ
D.ИљОнНЙбЧСђЫсФЦгыЫсЗДгІЕФаджЪЗжЮіЮѓВюЁЃ
(1)ЪЕбщжаШчЙћSO2ЭЈШыЙ§ЖрЃЌгЩгкSO2ЪЧЫсадбѕЛЏЮяЃЌПЩвдгыH2OЗДгІВњЩњH2SO3ЃЌЕМжТШмвКЫсаддіЧПЃЌЪЙНЙбЧСђЫсФЦгыH+ЗДгІЩњГЩSO2ЃЌгАЯьНЙбЧСђЫсФЦЕФжЦШЁЃЛ
(2)n(I2)=0.1000mol/LЁС0.04L=4.0ЁС10-3molЃЌn(Na2S2O3)=0.2000mol/LЁС0.02L=4.0ЁС10-3molЃЌдђИљОн2S2O32-+I2 =S4O62-+2I-ПЩжЊЪЃгрI2ЕФЮяжЪЕФСПЮЊn(I2)ЪЃ=![]() n(Na2S2O3)=
n(Na2S2O3)=![]() ЁС2.0ЁС10-3mol=2.0ЁС10-3molЃЌдђгыНЙбЧСђЫсФЦЗДгІЕФI2ЕФЮяжЪЕФСПЮЊ4.0ЁС10-3mol-2.0ЁС10-3mol=2.0ЁС10-3molЃЌИљОнЗНГЬЪНS2O52-+2I2+3H2O=2SO42-+4I-+6H+ПЩжЊдк0.2000 gВњЦЗжаКЌгаЕФНЙбЧСђЫсФЦЕФЮяжЪЕФСПn(Na2S2O5)=
ЁС2.0ЁС10-3mol=2.0ЁС10-3molЃЌдђгыНЙбЧСђЫсФЦЗДгІЕФI2ЕФЮяжЪЕФСПЮЊ4.0ЁС10-3mol-2.0ЁС10-3mol=2.0ЁС10-3molЃЌИљОнЗНГЬЪНS2O52-+2I2+3H2O=2SO42-+4I-+6H+ПЩжЊдк0.2000 gВњЦЗжаКЌгаЕФНЙбЧСђЫсФЦЕФЮяжЪЕФСПn(Na2S2O5)=![]() n(Na2S2O5)=
n(Na2S2O5)=![]() ЁС2.0ЁС10-3mol=1.0ЁС10-3molЃЌm(Na2S2O5)= 1.0ЁС10-3molЁС190g/mol=0.190gЃЌЫљвдНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ЮЊЃК
ЁС2.0ЁС10-3mol=1.0ЁС10-3molЃЌm(Na2S2O5)= 1.0ЁС10-3molЁС190g/mol=0.190gЃЌЫљвдНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ЮЊЃК![]() ЁС100%=95.00%ЃЛ
ЁС100%=95.00%ЃЛ
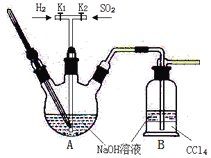
(3)A.SO2гыNaOHШмвКдкAжажЦШЁНЙбЧСђЫсФЦЃЌЮДЗДгІЕФSO2дкBзАжУжаБЛNaOHШмвКЮќЪеЃЌгЩгкSO2взгыNaOHЗДгІЃЌПЩИљОнCCl4ЕФУмЖШБШЫЎДѓЃЌВЛШмгкЫЎЃЌSO2вВВЛЗДгІЕФаджЪЃЌдкзАжУжаЗХжУCCl4ЃЌетбљОЭМШПЩвдЪЙSO2БЛГфЗжЮќЪеЃЌЭЌЪБгжЗРжЙЕЙЮќЯжЯѓЕФЗЂЩњЃЌAе§ШЗЃЛ
B.Na2S2O3ЪЧЧПМюШѕЫсбЮЃЌЫЎШмвКЯдМюадЃЌЫљвдЕЮЖЈЪБЃЌNa2S2O3ШмвКгІИУгУМюЪНЕЮЖЈЙмЪЂЗХЃЌBе§ШЗЃЛ
C.ЕЮЖЈжеЕуЕФЯжЯѓЪЧЙ§СПЕФБъзМI2ШмвКЧЁКУЗДгІЭъШЋЃЌШмвКгЩзиЛЦЩЋИеКУБфЮЊЮоЩЋЃЌCДэЮѓЃЛ
D.ШєЕЮЖЈЪБМфЙ§ГЄЃЌЕЮЖЈЗДгІВњЩњЕФH+ЛсгыЮДЕЮЖЈЕФNa2S2O3ЗДгІВњЩњSO2ЦјЬхвнГіЃЌЕМжТВњЦЗжаНЙбЧСђЫсФЦЕФжЪСПЗжЪ§ЦЋЕЭЃЌDДэЮѓЃЛ
ЙЪКЯРэбЁЯюЪЧCDЁЃ

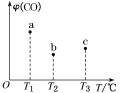
ЁОЬтФПЁПвбжЊСђДњСђЫсФЦдкЫсадЬѕМўЯТЛсЗЂЩњЗДгІЃК![]() ЃЌЯТБэжаЕФСНжжШмвКЛьКЯЃЌГіЯжЛызЧЕФЯШКѓЫГађЪЧЃЈ ЃЉ
ЃЌЯТБэжаЕФСНжжШмвКЛьКЯЃЌГіЯжЛызЧЕФЯШКѓЫГађЪЧЃЈ ЃЉ
зщКХ | СНжжШмвКЕФЮТЖШ |
| ЯЁСђЫсЕФЬхЛ§ЁЂХЈЖШ |
Ђй | 15Ёц | 10mL0.1mol/L | 50mL0.05mol/L |
Ђк | 15Ёц | 10mL0.05mol/L | 10mL0.1mol/L |
Ђл | 25Ёц | 10mL0.05mol/L | 10mL0.1mol/L |
Ђм | 25Ёц | 10mL0.1mol/L | 30mL0.07mol/L |
A.ЂмЂйЂкЂлB.ЂлЂмЂкЂй
C.ЂмЂлЂкЂйD.ЂмЂлЂйЂк