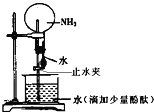
��Ŀ����
����Ŀ��25 ��ʱ���������ʵĵ��볣�����±���ʾ��
��ѧʽ | CH3COOH | H2C2O4 | H2S |
���볣�� | 1.8��10��5 | Ka1��5.4��10��2 Ka2��5.4��10��5 | Ka1��1.0��10��7 Ka2��7.1��10��15 |
��ش��������⣺
��1������CH3COOH��H2C2O4��HC2O4-��H2S��HS- ����������,����������������________��
��2��CH3COOH��H2C2O4��H2S��������ǿ������˳��Ϊ_____________________��
��3��NaHS��Һ��NaHC2O4��Һ��Ӧ�����ӷ���ʽΪ_______________��
��4��0.1 mol��L��1H2S��Һ��c(H+)=________ mol��L��1��
���𰸡�HS- H2C2O4>CH3COOH>H2S HS����HC2O![]() ===H2S����C2O
===H2S����C2O![]() 1.0��10��4
1.0��10��4
��������
ͬһ�¶��£���ĵ��볣��Խ��������Խǿ��������ĵ��볣��֪������������ǿ��˳���ǣ�H2C2O4��CH3COOH��HC2O4-��H2S��HS-������ǿ���Ʊ�����Ĺ��ɷ���NaHS��Һ��NaHC2O4��Ӧ�IJ������H2S�ĵ���ƽ�ⳣ�����м��㣨�Ե�һ��Ϊ������
��1��ͬһ�¶��£���ĵ��볣��Խ��������Խǿ��������ĵ��볣��֪������������ǿ��˳���ǣ�H2C2O4��CH3COOH��HC2O4-��H2S��HS-������������ǿ����H2C2O4����������HS-��
�ʴ��ǣ�HS- ��
��2��ͬһ�¶��£���ĵ��볣��Խ��������Խǿ��������ĵ��볣��֪������������ǿ��˳���ǣ�H2C2O4��CH3COOH��HC2O4-��H2S��HS-������CH3COOH��H2C2O4��H2S��������ǿ������˳��ΪH2C2O4��CH3COOH��H2S��
�ʴ��ǣ�H2C2O4>CH3COOH>H2S��
��3����������HC2O4-��H2S��HS-������NaHS��Һ��NaHC2O4��Һ��Ӧ����H2S��C2O![]() �����ӷ���ʽΪ��HS����HC2O
�����ӷ���ʽΪ��HS����HC2O![]() ===H2S����C2O
===H2S����C2O![]() ��
��
�ʴ��ǣ�HS����HC2O![]() ===H2S����C2O
===H2S����C2O![]() ��
��
��4��H2S�������ᣬ����̶Ƚ�С���Ե�һ������Ϊ���� ��֪H2S![]() HS-+H+��Ka1��1.0��10��7����0.1 mol��L��1H2S��Һ�У�������Ũ��C(H+)=x mol��L��1������ݵ���ƽ�ⳣ����֪��Ka1= C(H+) C(HS-)/c��H2S��������C(H+) ��C(HS-)�����ԣ�C2(H+)/0.1=1.0��10��7��C(H+)=10-4 mol��L��1��
HS-+H+��Ka1��1.0��10��7����0.1 mol��L��1H2S��Һ�У�������Ũ��C(H+)=x mol��L��1������ݵ���ƽ�ⳣ����֪��Ka1= C(H+) C(HS-)/c��H2S��������C(H+) ��C(HS-)�����ԣ�C2(H+)/0.1=1.0��10��7��C(H+)=10-4 mol��L��1��
�ʴ��ǣ�1.0��10��4��

 ��У����ϵ�д�
��У����ϵ�д�