��Ŀ����
����Ŀ����֪25�� Cʱ����Ԫ��H2X�ĵ���ƽ�ⳣ��K1=5.0��10-2��K2=5.4��10-5�����¶�����AgNO3��Һ�ֱ�ζ�Ũ�Ⱦ�Ϊ0.01mol��L-1��KY��K2X��Һ�����õij�����AgY��Ag2X���ܽ�ƽ��ͼ����ͼ��ʾ������������ȷ����
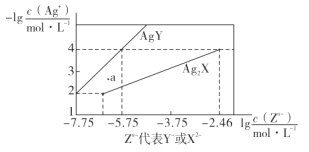
A.25��Cʱ��KHX����ҺpH>7
B.��ӦAg2X+2Y- 2AgY+ X2-��ƽ�ⳣ��Ϊ103.29
C.a��ȿ��Ա�ʾAgY�IJ�������ҺҲ���Ա�ʾAg2X�Ĺ�������Һ
D.��c(Y-)=c(X2-)=0.01 mol��L-1�Ļ��Һ�е���AgNO3��Һʱ��������AgY����
���𰸡�D
��������
���������ܽ�ƽ��ͼ���֪��������ΪAg+Ũ�ȵĸ����������������ֵԽ��Ag+��Ũ��ԽС��������ΪY-��X2-��Ũ�ȵĶ���ֵ���������Խ��Y-��X2-��Ũ��Խ����ͼ��֪����![]() ʱ��Ksp(AgY)=
ʱ��Ksp(AgY)=![]() ��ͬ�����ɵ�Ksp(Ag2X)=
��ͬ�����ɵ�Ksp(Ag2X)=![]() ���ݴ˽��з�����
���ݴ˽��з�����
A����HX-+H2O![]() H2X+OH-����֪����ˮ�ⳣ��Kh=
H2X+OH-����֪����ˮ�ⳣ��Kh=![]() ��K2��˵��KHX�Ե���Ϊ������25��Cʱ��KHX����ҺpH��7��A�����
��K2��˵��KHX�Ե���Ϊ������25��Cʱ��KHX����ҺpH��7��A�����
B���ɷ�����֪����ӦAg2X+2Y-![]() 2AgY+X2-��ƽ�ⳣ��ΪK=
2AgY+X2-��ƽ�ⳣ��ΪK=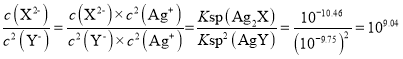 ��B�����
��B�����
C����ͼ��֪��a��ʱ��Q(AgY)��Ksp(AgY)����a���ʾAgY�Ĺ�������Һ��ͬ����a��ʱ��Q(Ag2X)��Ksp(Ag2X)����a���ʾAg2X�IJ�������Һ��C�����
D����c(Y-)=c(X2-)=0.01 mol��L-1ʱ��������AgY��������c(Ag+)=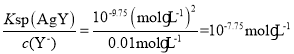 ��������Ag2X��������c(Ag+)=
��������Ag2X��������c(Ag+)=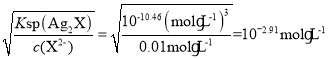 ��
��![]() 10-2.91mol��L-1>10-7.75mol��L-1���ɴ˿�֪����c(Y-)=c(X2-)=0.01 mol��L-1�Ļ��Һ�е���AgNO3��Һʱ��������AgY������D����ȷ��
10-2.91mol��L-1>10-7.75mol��L-1���ɴ˿�֪����c(Y-)=c(X2-)=0.01 mol��L-1�Ļ��Һ�е���AgNO3��Һʱ��������AgY������D����ȷ��
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��25 ��ʱ���������ʵĵ��볣�����±���ʾ��
��ѧʽ | CH3COOH | H2C2O4 | H2S |
���볣�� | 1.8��10��5 | Ka1��5.4��10��2 Ka2��5.4��10��5 | Ka1��1.0��10��7 Ka2��7.1��10��15 |
��ش��������⣺
��1������CH3COOH��H2C2O4��HC2O4-��H2S��HS- ����������,����������������________��
��2��CH3COOH��H2C2O4��H2S��������ǿ������˳��Ϊ_____________________��
��3��NaHS��Һ��NaHC2O4��Һ��Ӧ�����ӷ���ʽΪ_______________��
��4��0.1 mol��L��1H2S��Һ��c(H+)=________ mol��L��1��
����Ŀ������������ѧ��Ӧ��ƽ�ⳣ����K1��K2��K3�����¶ȵĹ�ϵ�ֱ����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
973K | 1173K | ||
��Fe��s��+CO2��g�� | K1 | 1.47 | 2.15 |
��Fe��s��+H2O��g�� | K2 | 2.38 | 1.67 |
��CO��g��+H2O��g�� | K3 | �� | �� |
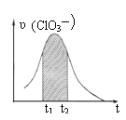
������˵����ȷ����
A����H1��0����H2��0
B����Ӧ�٢ڢ��ķ�Ӧ�������ϵ����H2����H1����H3
C����Ӧ�٢ڢ���ƽ�ⳣ�������ϵ��K1��K2��K3
D��Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ���´�ʩ
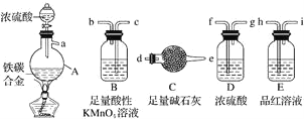
����Ŀ���й���ͼ��ʾʵ���Ԥ��������ȷ����
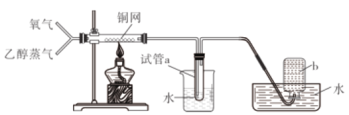
ѡ�� | ���� | Ԥ������ |
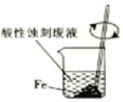
A | ͨ��һ��ʱ����������ȼ�ƾ��� | ͭ������� |
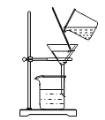
B | ͨ������������һ��ʱ���ͨ���Ҵ����� | ͭ������ɺ�ɫ |
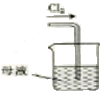
C | Ԥ��ͨ�������ž�װ���еĿ������ٽ���ʵ�� | ����ƿb�в����ռ������� |
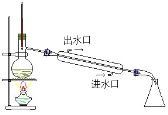
D | �۲��Թ�a���ռ�������Һ | ��Һ��ɫ�д̼�����ζ |
A.AB.BC.CD.D