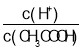��Ŀ����
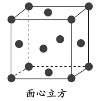
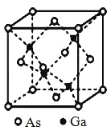
����Ŀ��GaAs ������۵�ܸߣ�Ӳ�Ⱥܴ��ܶ�Ϊ�� gcm��3��Ga�� As��Ħ�������ֱ�Ϊ MGa gmol��1 �� MAsgmol��1��ԭ�Ӱ뾶�ֱ�Ϊ rGa pm �� rAs pm�������ӵ�����ֵΪ NA���侧���ṹ��ͼ��ʾ������˵���������
A.�þ���Ϊ���۾���
B.�þ����� Ga �� As ���¶Ե��ӣ�Ga �� As ����λ����Ϊ 4
C.ԭ�ӵ����ռ��������İٷ���Ϊ![]()
D.����ԭ�Ӿ����� 8 �����ȶ��ṹ
���𰸡�C
��������
A. GaAs������۵�ܸߣ�Ӳ�Ⱥܴ�Ϊ�ռ�������״�ṹ�����ڹ��۾��壬��A��ȷ��
B. �ɾ����ṹ��֪��Ga����λ��Ϊ4��������Gaԭ����ĿΪ4��Asԭ����ĿΪ![]() ��������As��Gaԭ����Ŀ֮��Ϊ1��1����As��λ��Ҳ��4��Ga����Χ4��Asԭ���γ���������ṹ��As����Χ4��Gaԭ��Ҳ�γ���������ṹ��ԭ�Ӿ��γ�4������Gaԭ�Ӽ۵�����Ϊ3����As�γ�4�����ۼ���˵��Asԭ���ṩ1�Թµ��ӶԸ�Ga�γ���λ����Asԭ�������5������ȫ���ɼ�����û�й¶Ե��ӣ���B��ȷ��
��������As��Gaԭ����Ŀ֮��Ϊ1��1����As��λ��Ҳ��4��Ga����Χ4��Asԭ���γ���������ṹ��As����Χ4��Gaԭ��Ҳ�γ���������ṹ��ԭ�Ӿ��γ�4������Gaԭ�Ӽ۵�����Ϊ3����As�γ�4�����ۼ���˵��Asԭ���ṩ1�Թµ��ӶԸ�Ga�γ���λ����Asԭ�������5������ȫ���ɼ�����û�й¶Ե��ӣ���B��ȷ��
C. ������ԭ�������Ϊ![]() ����������Ϊ
����������Ϊ![]() �����������Ϊ
�����������Ϊ![]() ��ԭ�ӵ����ռ��������İٷ���Ϊ
��ԭ�ӵ����ռ��������İٷ���Ϊ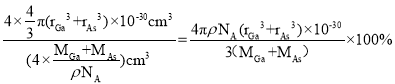 ����C����
����C����
D. ÿ��As��Gaԭ�Ӷ��γ�4�����ۼ�����û�й¶Ե��ӣ�����ԭ�Ӿ�����8�����ȶ��ṹ����D��ȷ��
�ʴ�ѡ��C��

����Ŀ���о�����CO2�ŷ���һ����Ҫ���⡣CO2��������������ɵ�̼�л����Ҫ�����·�Ӧ��
��Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1=��49.6kJ/mol
CH3OH(g)��H2O(g) ��H1=��49.6kJ/mol
��Ӧ��CH3OCH3(g)��H2O(g)![]() 2CH3OH(g) ��H2����23.4kJ/mol
2CH3OH(g) ��H2����23.4kJ/mol
��Ӧ��2CO2(g)��6H2(g)![]() CH3OCH3(g)��3H2O(g) ��H3
CH3OCH3(g)��3H2O(g) ��H3
��1����H3��_______��
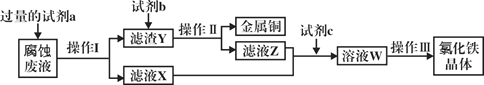
��2�����º��������£����ܱ�������ͨ������ʵ�����CO2��H2��������ӦI������������˵����ӦI�ﵽƽ��״̬����_______������ţ���
A.��Ӧ��ϵ��ѹǿ���ֲ���
B.�����ڵĻ��������ܶȱ��ֲ���
C.ˮ���Ӷ���2NA��H-O����ͬʱ����Ӷ���3NA��H-H��
D.CH3OH��H2O��Ũ��֮�ȱ��ֲ���
��3����ӦII��ij�¶��µ�ƽ�ⳣ��Ϊ0.25�����¶��£����ܱ������м�������ʵ�����CH3OCH3(g)��H2O(g)����Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OCH3(g) | H2O(g) | CH3OH(g) |
Ũ��/mol��L-1 | 1.8 | 1.8 | 0.4 |
��ʱv��_______v������������������������������������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH�������(CH3OH)%=_______%��
��4����ijѹǿ�£���ӦIII�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ƽ��ת������ͼ��ʾ��T1�¶��£���6molCO2��12molH2����2L���ܱ������У�5min��Ӧ�ﵽƽ��״̬����0��5min�ڵ�ƽ����Ӧ����v(CH3OCH3)=_______��KA��KB��KC����֮��Ĵ�С��ϵΪ________��
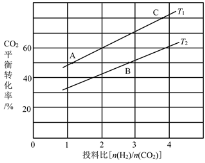
��5����ѹ�½�CO2��H2�������1��3��ϣ��ڲ�ͬ���������·�����ӦI�ͷ�ӦIII������ͬ��ʱ�����CH3OH��ѡ���ԺͲ������¶ȵı仯��ͼ�����У�CH3OH��ѡ����=![]() ��100%��
��100%��
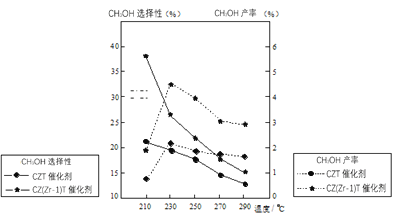
���¶ȸ���230�棬CH3OH�������¶����߶��½���ԭ����______��
�������������ºϳɼ״��Ĺ�ҵ������_______��
A.210�� B.230�� C.����CZT D.����CZ(Zr��1)T
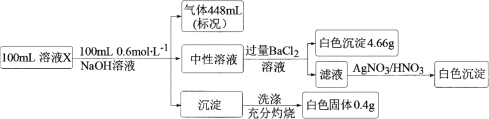
��6��CO2���Ա�(NH4)2CO3��Һ����ӦΪ(NH4)2CO3(aq)��H2O(l)��CO2(g)=2NH4HCO3(aq)��Ϊ�о��¶ȶ�(NH4)2CO3����CO2 Ч�ʵ�Ӱ�죬��ij�¶�T1�£���һ������(NH4)2CO3��Һ�����ܱ������У�������һ������CO2����(�õ�����Ϊϡ�ͼ�)����tʱ�̣����������CO2�����Ũ�ȡ�Ȼ��ֱ����¶�ΪT2��T3��T4��T5�£�����������ʼʵ���������䣬�ظ�����ʵ�飬������ͬʱ����CO2����Ũ�ȣ����ϵ��ͼ������H______0(����>��������������<��)��