��Ŀ����
����Ŀ����һ�������£���4molNH3��4molO2����ڹ̶��ݻ�Ϊ2L���ܱ������У�������Ӧ��4NH3(g)+5O2(g)=4X(g)+6H2O(g)��2min��÷�Ӧ�ﵽƽ�⣬����3molH2O����
(1)X�Ļ�ѧʽΪ___��
(2)O2��ת����Ϊ___(O2ת����=�ѷ�Ӧ��O2����/O2��������100%)��
(3)0~2min�ڣ�v(NH3)=___mol��L-1��min-1��
(4)ȼ�ϵ����һ�ָ�Ч�������Ѻ��ͷ���װ�á�һ��ȼ�ϵ�صĵ������ҺΪNaOH��Һ������ͨ��NH3������ͨ�����������Ի�������Ⱦ�����ĵ缫��ӦʽΪ___����·��ÿͨ��1mol���ӣ����ı�״���µĿ���___(���������O2�ĺ���Ϊ20%)L��
���𰸡�NO 62.5%(��0.625��![]() ) 0.5 2NH3��6e-+6OH-
) 0.5 2NH3��6e-+6OH-![]() N2+6H2O 28
N2+6H2O 28
��������
(1)����Ԫ���غ��֪X�Ļ�ѧʽΪNO��
(2)���ݷ���ʽ��֪������3molˮ����2.5mol����������������ת����Ϊ![]() =62.5%��
=62.5%��
(3)0~2min�ڣ�v(H2O)=![]() =0.75 mol��L-1��min-1��ͬһ��Ӧ��ͬ���ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����v(NH3)=
=0.75 mol��L-1��min-1��ͬһ��Ӧ��ͬ���ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����v(NH3)=![]() v(H2O)=0.5 mol��L-1��min-1��
v(H2O)=0.5 mol��L-1��min-1��
(4)����ͨ��NH3��ԭ����и�������������Ӧ����������Ⱦ�����Բ���ΪN2���������Һ�Լ��ԣ�����ͬʱ����ˮ�����ݵ����غ��Ԫ���غ�ɵõ缫����ʽΪ2NH3��6e-+6OH-��N2+6H2O����Ӧ������O2��OH-������ÿ���������Եõ�4�����ӣ���·��ÿͨ��1mol���ӣ�������0.25mol������������O2�ĺ���Ϊ20%����������1.25mol���������Ϊ1.25mol��22.4L/mol=28L��

����Ŀ���������������ܰ뵼�����Ҫԭ�ϡ���ҵ�ϳ���п��ұ���ķ����л����ء���֪ijп������Ҫ��Zn��Si��Pb��Fe��Ga����������øÿ������صĹ����������£�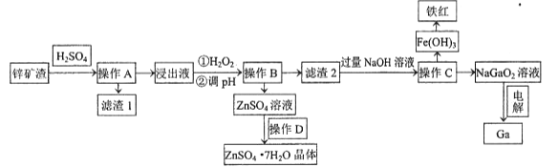
��֪��������Ԫ�����ڱ���λ�ڵ������ڵڢ�A�壬��ѧ�����������ơ�
��lg2=0.3 lg3=0.48��
�۲������ʵ�Ksp���±���ʾ��
���� | Zn(OH)2 | Ga(OH)3 | Fe(OH)2 | Fe(OH)3 |
Ksp | 1.6��10��17 | 2.7��10��31 | 8��10��16 | 2.8��10��39 |
(1)Ϊ�����������ʣ����ʵ���������Ũ���⣬Ӧ��ȡ�Ĵ�ʩ��__________________��д��������������1����Ҫ�ɷ�������Ǧ��_________________(д��ѧʽ)��
(2)����H2O2��Ŀ����(�����ӷ���ʽ��ʾ)______________________ ��
(3)���������£�������Һ�и������ӵ�Ũ�Ⱦ�Ϊ0.01mo/L������Һ��ij������Ũ��С��1��10-5mol/Lʱ����Ϊ����������ȫ��ȥ����pHӦ���ڵķ�ΧΪ___________________��
(4)����D������_______________���ˡ�ϴ�ӡ����
(5)��ⷨ�Ʊ������ء��ö��Ե缫���NaGaO2��Һ�����Ƶý����أ�д�������缫��Ӧʽ________________________��