题目内容
【题目】图甲中①②③④表示不同化学元素所组成的化合物,图乙表示由四个单体构成的化合物。以下说法不正确的是( )
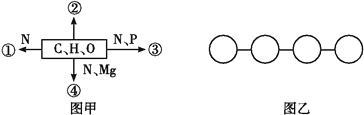
A. 若图甲中的②大量存在于皮下和内脏器官周围等部位,则②是脂肪
B. 若图甲中④能吸收、传递和转换光能,则④可用无水乙醇提取
C. 图乙中若单体是氨基酸,则该化合物彻底水解后的产物中氧原子数增加3个
D. 图乙中若单体是四种脱氧核苷酸,则该化合物彻底水解后的产物有5种
【答案】D
【解析】
②的组成元素只有C、H、O,且存在于皮下和内脏器官周围等部位,可能是脂肪,A正确;④的组成元素是C、H、O、N、Mg,能吸收、传递和转换光能,则④可能是叶绿素,可用无水乙醇提取,B正确;乙图中若单体是氨基酸,则为四肽,则该化合物彻底水解需要3分子水,所以水解后的产物中氧原子数增加3个,C正确;乙图中若单体是四种脱氧核苷酸,则为DNA,该化合物彻底水解后的产物有脱氧核糖、磷酸和四种碱基A、C、G、T,共6种,D错误。

 华东师大版一课一练系列答案
华东师大版一课一练系列答案【题目】已知2A(g)+B(g)![]() 2C(g) ΔH=-a kJ·mol-1(a>0),在一个有催化剂的固定容积的容器中加入2 mol A和1 mol B,在500 ℃时充分反应达平衡后,C的浓度为ω mol·L-1,放出的热量为b kJ。
2C(g) ΔH=-a kJ·mol-1(a>0),在一个有催化剂的固定容积的容器中加入2 mol A和1 mol B,在500 ℃时充分反应达平衡后,C的浓度为ω mol·L-1,放出的热量为b kJ。
(1)已知:A(g)+X(g)![]() 2B(g) ΔH=-133.2 kJ·mol-1;
2B(g) ΔH=-133.2 kJ·mol-1;
5A(g)+X(g)![]() 4C(g) ΔH=-650.4 kJ·mol-1。则a=________。
4C(g) ΔH=-650.4 kJ·mol-1。则a=________。
(2)不同温度下该反应的平衡常数如表所示。由此可推知,表中T1________T2(填“>”“=”或“<”)。
T/K | T1 | T2 | T3 |
K | 1.00×107 | 2.45×105 | 1.88×103 |
若在原来的容器中,只加入2 mol C,500 ℃时充分反应达平衡后,吸收热量为c kJ,C的浓度________________ω mol·L-1(填“>”“=”或“<”),a、b、c之间的关系为________________。
(3)在相同条件下要想得到2a kJ热量,加入各物质的物质的量可能是________(填序号)。
A.4 mol A和2 mol B
B.4 mol A、2 mol B和2 mol C
C.4 mol A和4 mol B
D.6 mol A和4 mol B
(4)若将上述容器改为恒压容器(反应前体积相同),起始时加入2 mol A和1 mol B,500 ℃时充分反应达平衡后,放出的热量为d kJ,则d________b(填“>”“=”或“<”),理由是____________________________________。
(5)在一定温度下,向一个容积可变的容器中,通入3 mol A和2 mol B及固体催化剂,充分反应,平衡时容器内气体物质的量为起始时的90%。保持同一反应温度,在相同容器中,将起始物质的量改为4 mol A、3 mol B和2 mol C,则平衡时A的百分含量________(填“不变”“变大”“变小”或“无法确定”)。