��Ŀ����
10����1��ijԪ�ص�ͬλ��X�����Ȼ���XCl2��1.11g����ˮ�Ƴ���Һ����1mol/L��AgNO3��Һ20mLǡ����ȫ��Ӧ��������ͬλ��ԭ�Ӻ�����20�����ӣ���Ԫ��X��Zֵ��Aֵ����2����ij100 mL���Һ�У�HNO3��H2SO4�����ʵ���Ũ�ȷֱ���0.4 mol/L��0.1 mol/L����û��Һ�м���1.92 gͭ�ۣ����ȣ�����ַ�Ӧ��������Һ�е�Cu2+�����ʵ���Ũ���Ƕ��٣�
���� ��1����Cl-+Ag+�TAgCl������XCl2�����ʵ���������M=$\frac{m}{n}$������Ħ��������Ħ����������Է�����������ֵ��ȣ�ԭ�ӵ�������Ϊԭ�ӵĽ������ԭ����������ϸ�ԭ��ԭ�Ӻ�����20�����ӣ�����������=������-������������ԭ�ӵ���������
��2��n��Cu��=$\frac{1.92g}{64g/mol}$=0.03mol��n��H+��=0.4mol/L��0.1L+0.1mol/L��2��0.1L=0.06mol��n��NO3-��=0.4mol/L��0.1L=0.04mol������3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���жϹ������Բ�����������㣮
��� �⣺��1����Cl-+Ag+�TAgCl����֪��n��Cl-��=n��Ag+��=0.02L��1mol/L=0.02mol��
n��XCl2��=$\frac{1}{2}$n��Cl-��=$\frac{1}{2}$��0.02mol=0.01mol��
��M��XCl2��=$\frac{1.11g}{0.01mol}$=111g/mol��
����XCl2��Է�������Ϊ111��X�����ԭ������=111-35.5��2=40����X��������Ϊ40��
������=������-������=40-20=20������Z=20��A=40��
��Ԫ��X��Zֵ��Aֵ�ֱ�ΪZ=20��A=40��
��2��n��Cu��=$\frac{1.92g}{64g/mol}$=0.03mol��n��H+��=0.4mol/L��0.1L+0.1mol/L��2��0.1L=0.06mol��n��NO3-��=0.4mol/L��0.1L=0.04mol��
�� 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
3 8 2
0.03mol 0.08mol 0.02mol
��Ȼ�����ӵ����ʵ������㣬
�������ӵ����ʵ��������ӷ���ʽ��֪0.06mol�����ӷ�Ӧ�����ɵ�ͭ���ӵ����ʵ���Ϊ0.06mol��$\frac{3}{8}$=0.0225mol��
������Һ��c��Cu2+��=$\frac{0.0225mol}{0.1L}$=0.225mol/L��
��������Һ�е�Cu2+�����ʵ���Ũ��Ϊ0.225mol/L��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ������Ӧ��ԭ��Ϊ���ؼ�����2��Ϊ�״��㣬��Ҫ�������ӷ���ʽ������Ӧ������жϹ��������ݲ��������㣮

| ��һ�� | He | -268.8 | ��a�� | -249.5 | Ar | -185.8 | Kr | -151.7 |
| �ڶ��� | F2 | -187.0 | Cl2 | -33.6 | ��b�� | 58.7 | I2 | 184.0 |
| ������ | ��c�� | 19.4 | HCl | -84.0 | HBr | -67.0 | HI | -35.3 |
| ������ | H2O | 100.0 | H2S | -60.2 | ��d�� | -42.0 | H2Te | -1.8 |
| A�� | abc�Ļ�ѧʽ�ֱ�ΪNe2��Br2��HF | |
| B�� | ���������������Ƚϣ���������ȶ�˳��Ϊ��HBr��d | |
| C�� | ��������������ˮ����Һ������c��ǿ | |
| D�� | ������������H2O�ķе���ߣ�����ΪH2O�����ڴ������ |
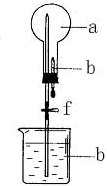 ����ͼ��ʾ��װ���У���ƿ�г�����������a�����ι��е�Һ��b������ƿ�ڣ���������ƿ��Ȼ����ɼ�f���ձ��е�Һ��b����Ȫ״��������ռ���������ƿ����a��b��������ĿҪ����ǣ�������
����ͼ��ʾ��װ���У���ƿ�г�����������a�����ι��е�Һ��b������ƿ�ڣ���������ƿ��Ȼ����ɼ�f���ձ��е�Һ��b����Ȫ״��������ռ���������ƿ����a��b��������ĿҪ����ǣ�������| a���������壩 | b��Һ�壩 | |
| A | NO2 | ˮ |
| B | CO2 | 4mol/LNaOH��Һ |
| C | Cl2 | ����NaOH��Һ |
| D | NH3 | 1mol/L���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
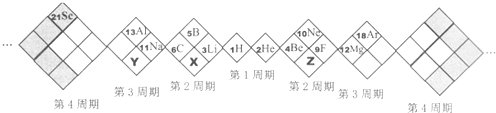
| A�� | X��Y��ZԪ�طֱ�ΪN��P��O | B�� | ����Ԫ�ض�������Ԫ�� | ||
| C�� | ԭ�Ӱ뾶��Z��X��Y | D�� | �ȶ��ԣ�X���⻯�Y���⻯�� |
| A�� | ���������V����V����������V������������V�������� | |
| B�� | ���������V����V���������仯���ұ仯�ı�����ͬ | |
| C�� | ����ѹǿʱ��V����V����������V������������V�������� | |
| D�� | �����¶�ʱ��V����V������С����V����С����С��V����С���� |