��Ŀ����
1��ij�о�С��ģ�ҵ���Ի�����Ϊԭ���Ʊ�����ĵ�һ����Ӧ���£�4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2 ��������ʵ�飬���ⶨ����Ʒ��FeS2��Ʒ�Ĵ��ȣ������������ʲ����뷴Ӧ����
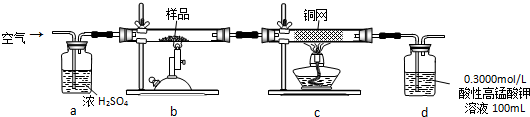
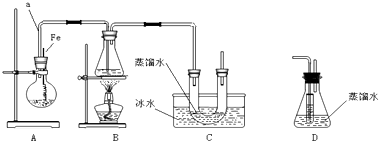
ʵ�鲽�裺��ȡ��ϸ����Ʒ4.000g������ͼbװ���У�Ȼ���ڿ����н������գ�Ϊ�ⶨδ��Ӧ������ص�������������Һ������ֲ��䣩��ʵ����ɺ�ȡ��d����Һ10mL������ƿ���0.1000mol/L���ᣨH2C2O4������Һ���еζ���
����֪��5SO2+2KMnO4+2H2O�TK2SO4+2MnSO4+2H2SO4��
��ش��������⣺
��1��������Ʒ�����ܷ���������ƽ���ܣ���ܡ����ܡ�����ȡ��d����Һ10mL��Ҫ��Dȷ��ȡ������ţ�
A������ƿ B����Ͳ C����ʽ�ζ��� D����ʽ�ζ���
��2��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ���Ǵٽ�װ���еĶ�����������ȫ������
��3����֪������������������Һ������CO2��Mn2+���������ɣ���ζ�ʱ������Ӧ�����ӷ���ʽΪ2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O�r�жϵζ������յ�ķ����ǵ������һ�β�����Һ����Һ���Ϻ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
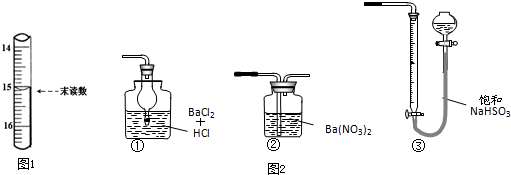
��4����֪�ζ��ܳ�����Ϊ0.10mL��ĩ������ͼ1��ʾ�����IJ�����Һ�����Ϊ15.00mL��
���в����ᵼ�¸���Ʒ��FeS2�Ĵ��Ȳⶨ���ƫ�ߵ���C������ţ���
A��ʢ����Һ�ĵζ���������ˮϴ�Ӻ�δ�ñ�Һ��ϴ��װҺ�ζ�
B����ƿ������ˮϴ�Ӻ�δ�ô���Һ��ϴ
C����ȡ��Һ����ʱ���ζ�ǰƽ�ӣ��ζ����յ����
D���ζ�ǰ�ζ��ܼ��촦������δ�ų����ζ���������ʧ
��5������Ʒ��FeS2�Ĵ���Ϊ90%��
��6������ͼ2װ���������ʵ��װ��d��ͬ�����Դﵽʵ��Ŀ���Ǣڣ����ţ���
���� ������ͨ��Ũ�����н����������b��������������������ӦΪ4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��Ȼ��������ͨ��c�У������ķ�ӦΪ2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��Ȼ������ͨ��d�У����������ܱ����������Һ�����������ᣬ
��1��������Ʒ��������Ϊ0.001g��������ƽ����Ϊ0.1g��
d����Һ�����ԣ�Ӧ������ʽ�ζ�����ȡ��
��2�����������ж�����ֱ���ſգ�����ʵ����Ӧ�ý�װ���ж��������ž���
��3��������������������Һ������CO2��Mn2+���������ɣ���ζ�ʱ������Ӧ�����ӷ���ʽΪ 2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O�r�жϵζ������յ�ķ����ǵ������һ�β�����Һ����Һ���Ϻ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
��4�����ݵζ��ܵĽṹ�����ÿ�ʼ�ͽ����������ֵ����õ����IJ�����Һ�������
�ζ����������ʹ�õIJ������ƫ����ⶨ��������������������ƫС��
��5���������շ�Ӧ�͵ζ���Ӧ�Ķ�����ϵ����������������������
��6��װ��d���������ղ��ⶨ�����ж�������ĺ��������ݶ�������Ļ�ѧ���ʽ����жϣ�
��� �⣺������ͨ��Ũ�����н����������b��������������������ӦΪ4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��Ȼ��������ͨ��c�У������ķ�ӦΪ2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��Ȼ������ͨ��d�У����������ܱ����������Һ�����������ᣬ
��1��������Ʒ��������Ϊ0.001g��������ƽ����Ϊ0.1g�����Բ��ܴ��棻
d����Һ�����ԣ�Ӧ������ʽ�ζ�����ȡ����ѡD��
�ʴ�Ϊ�����ܣ�D��
��2�����������ж�����ֱ���ſգ�����ʵ����Ӧ�ý�װ���ж��������ž����������Ⱦ�������ʴ�Ϊ���ٽ�װ���еĶ�����������ȫ�����գ�
��3��������������������Һ������CO2��Mn2+���������ɣ���ζ�ʱ������Ӧ�����ӷ���ʽΪ 2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O�r�жϵζ������յ�ķ����ǵ������һ�β�����Һ����Һ���Ϻ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
�ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O���������һ�β�����Һ����Һ���Ϻ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
��4�����ݵζ��ܵĽṹ�����ÿ�ʼ�ͽ����������ֵ����õ����IJ�����Һ�����Ϊ��15.10mL-0.10mL=15.00mL��
A��ʢ����Һ�ĵζ���������ˮϴ�Ӻ�δ�ñ�Һ��ϴ��װҺ�ζ������²���Ũ�Ƚ��ͣ���ʹ�õIJ������ƫ����δ���������Ӧ�ĸ���������ʵ���ƫ���������ⶨֵƫ�ͣ��ʴ���
B����ƿ������ˮϴ�Ӻ�δ�ô���Һ��ϴ����Ӱ��ⶨ���ʴ���
C����ȡ��Һ����ʱ���ζ�ǰƽ�ӣ��ζ����յ���ӣ����²������ƫС��δ���������Ӧ����������ʵ���ƫ�ⶨֵƫ�ߣ�����ȷ��
D���ζ�ǰ�ζ��ܼ��촦������δ�ų����ζ���������ʧ�����²������ƫ��δ���������Ӧ�ĸ���������ʵ���ƫС���������IJⶨֵƫ�ͣ��ʴ���
�ʴ�Ϊ��15.00��C��
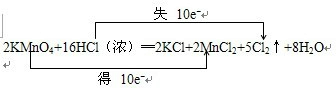
��5�����ݷ�Ӧ�����ӷ���ʽ�������������Ӧ�ĸ���������ʵ������õ�������������ʵ��������Ԫ���غ������������������������Ϸ�Ӧ�����ӷ���ʽ����õ���2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��100mL��Һ��ʣ�����������ʵ���Ϊ��$\frac{2}{5}$��0.01500L��0.1000mol/L��10=0.006mol�����������Ӧ�ĸ���������ʵ���Ϊ��0.3000mol/L��0.1000L-0.006mol=0.024mol����Ϸ�Ӧ5SO2+2KMnO4+2H2O�TK2SO4+2MnSO4+2H2SO4�ɵã�
5FeS2��10SO2��4KMnO4
5 4
n��FeS2�� 0.024mol
n��FeS2��=0.03mol
��Ʒ��FeS2�Ĵ���Ϊ��$\frac{0.03mol��120g/mol}{4.000g}$��100%=90%��
�ʴ�Ϊ��90%��
��6��ͼ2�У�ֻ�Т��ܹ����������Ӧ�������ᱵ�������������װ��d�����������ն��������п���������ˮ�������˶�������IJⶨ��
�ʴ�Ϊ���ڣ�
���� ���⿼��̽��������ɼ��京���ⶨ��Ϊ��Ƶ���㣬��ȷʵ��ԭ�����ζ������ǽⱾ��ؼ������ؿ���ѧ��ʵ�����������������Ӧ��������ע��ζ��̶ܿ�ֵ�ص㣬��Ŀ�Ѷ��еȣ�

| A�� | 14C��������������ļ�����14C��12C��Ϊͬ�������� | |
| B�� | Ѥ���ͷ��̻���ԭ�Ӻ�����ӷ���ԾǨ���������й� | |
| C�� | Ҷ���ء�Ѫ���غ�ά����B12��������� | |
| D�� | ����һ������X-����ͨ��ʯӢ����ʱ���ڼ�¼���Ͽɿ��������İߵ������ |
| A�� | ���뱽��ˮ�Ļ������÷�Һ�� | |
| B�� | ��Na2CO3��Һ�еõ�Na2C03�����ù��˷� | |
| C�� | ����ƾ���CCl4�Ļ������÷�Һ�� | |
| D�� | ����NaCl��AgCl�Ļ���������ȡ�� |
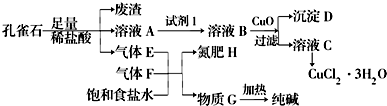
��֪����ҺAֻ��Cu2+��Fe2+��Fe3+���ֽ������ӣ����������ӳ���ʱ��pH�����ʾ���ش��������⣮
| �������� | Fe3+ | Fe2+ | Cu2+ | |
| pH | �������↑ʼ���� | 1.9 | 7.0 | 4.7 |
| �������ﰲȫ���� | 3.2 | 9.0 | 6.7 | |
��2��ͼ�С��Լ�1����ΪH2O2�����������еľ�������ΪFe2+��ʼ����ʱ��pHΪ7.0����ʱCu2+����ȫ��������������ȥCu2+�л��е�Fe2+������˫��ˮ��Ҳ�����������Ƚ�������ΪFe3+��
��3������CuO�������ǵ�����ҺpH����pH�ķ�ΧΪ3.2��pH��4.7��
��4������ҺC���CuCl2•3H2O����Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����ϴ��CuCl2•3H2O������Ҫ����Ҫ�����������ձ�����������©����
| t/s | 0 | 5 | 15 | 25 | 35 |
| n��A��/mol | 1.0 | 0.85 | 0.81 | 0.80 | 0.80 |
| A�� | ��Ӧ��ǰ5s��ƽ������v��A��=0.17mol•L-1•s-1 | |
| B�� | ���������������䣬�����¶ȣ�ƽ��ʱc��A��=0.41mol•L-1����Ӧ�ġ�H��0 | |
| C�� | ��ͬ�¶��£���ʼʱ�������г���2.0mol C���ﵽƽ��ʱ��C��ת���ʴ���80% | |
| D�� | ��ͬ�¶��£���ʼʱ�������г���0.20mol A��0.20mol B��1.0mol C����Ӧ�ﵽƽ��ǰv��������v���棩 |
 ��
��
 +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$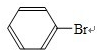 +HBr
+HBr