��Ŀ����
�Ȼ�ͭ��һ�ֹ㷺�����������ϡ���ľ�ķ������Ļ�����Ʒ��ij�о���ѧϰС���ô�ͭ��������Fe�������������Ʊ��Ȼ�ͭ���塣
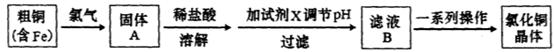
��1������A��ϡ�����ܽ������ˮ�ܽ��ԭ����________��
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ�X��ѡ�������Լ��е�____������ţ���
��3����ҺB��һϵ�в����ɵ��Ȼ�ͭ���壬�����ij�������Ϊ_________ ��_________�� ���ˡ���Ȼ���
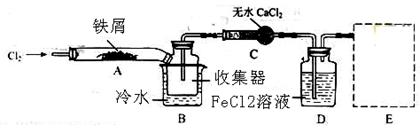
��4��ʵ���Ҳ�������ͼ��ʾװ�ã���ʹ��ͭ��Cl2��Ӧת��Ϊ����A�����ּ��������ͼг�װ������ȥ����
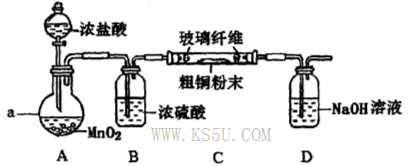
�ٸ�װ��������a��������____�����з�����Ӧ�����ӷ���ʽ��____________��
����ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊ�Ƿ��Ҫ��____________����ǡ�����
�۸�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ��_______________________��
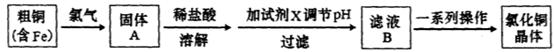
��1������A��ϡ�����ܽ������ˮ�ܽ��ԭ����________��
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ�X��ѡ�������Լ��е�____������ţ���
| A��NaOH | B��NH3.H2O | C��CuO | D��Cu(OH)2E. CuSO4 |
��4��ʵ���Ҳ�������ͼ��ʾװ�ã���ʹ��ͭ��Cl2��Ӧת��Ϊ����A�����ּ��������ͼг�װ������ȥ����

�ٸ�װ��������a��������____�����з�����Ӧ�����ӷ���ʽ��____________��
����ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊ�Ƿ��Ҫ��____________����ǡ�����
�۸�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ��_______________________��
��1������Cu2����Fe3�������ӷ���ˮ�ⷴӦ ��2��c d ��3������Ũ������ȴ�ᾧ
��4���� Բ����ƿ MnO2 + 4H++2Cl�� Mn2++ Cl2�� + 2H2O
Mn2++ Cl2�� + 2H2O
�� �� �� ��װ��C��D֮������һ��������װ��
��4���� Բ����ƿ MnO2 + 4H++2Cl��
 Mn2++ Cl2�� + 2H2O
Mn2++ Cl2�� + 2H2O �� �� �� ��װ��C��D֮������һ��������װ��
�����������1����ͭ�к���Cu��Fe����������Ӧʱ��ʱCuCl2��FeCl3�����ڶ��߶���ǿ�������Σ�������ˮ�ⷴӦ����Cu(OH)2��Fe(OH)3��Ϊ������Cu2����Fe3�������ӷ���ˮ�ⷴӦͨ����ϡ�������ܽ⡣
��2�����Լ�X���ڵ���pH�Գ�ȥ���ʣ��������������µ��������ӡ�������Ŀ���������ʣ���ѡ��CuO��Cu(OH)2��ѡ��ΪCD
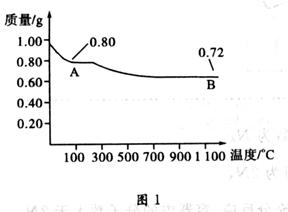
��3������ȥFe(OH)3�����ĺ���CuCl2��Һ��һϵ�в����ɵ��Ȼ�ͭ���塣CuCl2��ǿ�������Σ�ˮ�����Cu(OH)2��HCl�������лӷ��ԣ����ӷ��ݳ����õ��Ĺ�����Cu(OH)2�����������ܽ�����¶ȵ�Ӱ��仯�ϴ����Կɲ��õIJ����ij�������Ϊ����Ũ������ȴ�ᾧ�����ˡ���Ȼ�����4��ʵ������ȡCl2����������ƿ����Ũ������������̹��ȵķ�����ȡ�ġ���Ӧ����������ʽΪ��MnO2 + 4H++2Cl��
 Mn2++ Cl2�� + 2H2O������ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊû�б�Ҫ����ΪCu��HCl���ᷢ����Ӧ��ֻ��Cl2������Ӧ��������Cl2������NaOH������Ӧ�����µ������е�ѹǿ��С����ʱ�ձ��е���Һ������������װ�ã��ʸ�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ����װ��C��D֮������һ��������װ�á�
Mn2++ Cl2�� + 2H2O������ͬѧ��ΪӦ��Ũ����ϴ��ƿǰ��������HCI��װ�ã�����Ϊû�б�Ҫ����ΪCu��HCl���ᷢ����Ӧ��ֻ��Cl2������Ӧ��������Cl2������NaOH������Ӧ�����µ������е�ѹǿ��С����ʱ�ձ��е���Һ������������װ�ã��ʸ�װ�ô���һ���İ�ȫ�����������ð�ȫ�����Ĵ�ʩ����װ��C��D֮������һ��������װ�á�
��ϰ��ϵ�д�
�����Ŀ
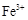 ��
�� Ũ����ȡ����ѷ�Ӧ��
Ũ����ȡ����ѷ�Ӧ��