��Ŀ����
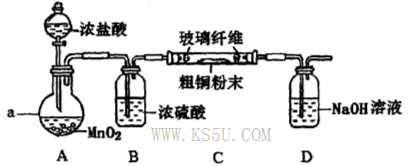
��10�֣�ij�Ȼ�����Ʒ����FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

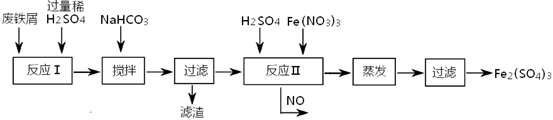
��1������I���õ��IJ����������ձ����������⣬��������________________________�����������ƣ���
��2��д��������ˮ������Ӧ�����ӷ���ʽ____________________��
��3����������Ѿ�ϴ�Ӹɾ��IJ�����������______________________________________
___________________________________________________________________________��
��4����������ΪW1g�����Ⱥ����������ɫ����������ΪW2g������Ʒ����Ԫ�ص�����������____________________���г�ԭʼ��ʽ�����軯��������ȷ�����ղ����Ľ��ƫ�����������ԭ�������_____________________��д��һ��ԭ�ɣ���

��1������I���õ��IJ����������ձ����������⣬��������________________________�����������ƣ���
��2��д��������ˮ������Ӧ�����ӷ���ʽ____________________��
��3����������Ѿ�ϴ�Ӹɾ��IJ�����������______________________________________
___________________________________________________________________________��
��4����������ΪW1g�����Ⱥ����������ɫ����������ΪW2g������Ʒ����Ԫ�ص�����������____________________���г�ԭʼ��ʽ�����軯��������ȷ�����ղ����Ľ��ƫ�����������ԭ�������_____________________��д��һ��ԭ�ɣ���

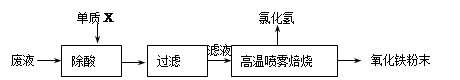
�����������1����Һ�����ƻ���Ҫ100ml����ƿ�ͽ���ιܣ���Ҫע���������ƿ����ע��100ml��
��2��������ˮ��Cl2��Fe2+����ΪFe3+��
��3�����δϴ�Ӹɾ����������溬��NH4Cl��������AgNO3��������һ��ϴ��Һ��Cl?���ɡ�
��4�����պ�õ��Ĺ���ΪFe2O3������ΪW2-W1�������FeԪ�ص�����Ϊ��112��W2-W1��/160,��Ϊԭ��Ʒ������100��00ml��Һ������ȡ����20��00ml���е�ʵ�飬���Գ���5��ԭ��Ʒ����Ԫ�ص������������������������

��ϰ��ϵ�д�
�����Ŀ
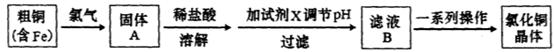
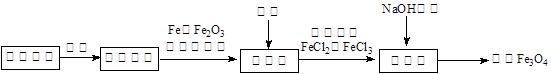

 4Fe3++2H2O
4Fe3++2H2O