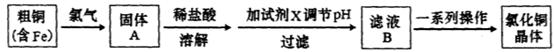��Ŀ����
FeCl3���ִ���ҵ������Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ���������Ʊ���ˮFeCl3�����ø���ƷFeCl3��Һ�����ж���H2S��
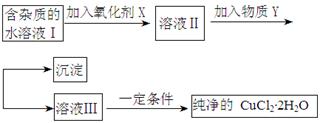
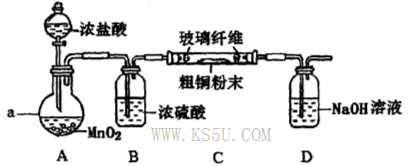
��.���������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮFeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�
�ټ��װ�õ������ԣ�
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ���
�ܣ���������������
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����N2�Ͼ�Cl2�����ռ����ܷ�
��ش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��2���ڢ۲����Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A�Ҷ�Ҫʹ������FeCl3�����ռ������ڢܲ������� ��
��3�����������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ� ��
��4��װ��D��FeCl2ȫ����Ӧ����ʧȥ����Cl2�����ö�ʧЧ��д������FeCl2�Ƿ�ʧЧ���Լ��� ��
��5�������߿��л���β������װ��E��ע���Լ���
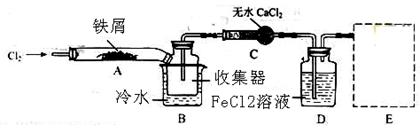
����ͬѧ��װ��D�еĸ���ƷFeCl3��Һ����H2S���õ��������˺�����ʯīΪ�缫����һ�������µ����Һ��
��6��FeCl3��H2S��Ӧ�����ӷ���ʽΪ ��
��7��������H+�������ŵ����H2�������ĵ缫��ӦʽΪ ��
��8���ۺϷ���ʵ����������Ӧ����֪��ʵ�������������ŵ㣺
��H2S��ԭ��������Ϊ100%���� ��
��.���������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮFeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�

�ټ��װ�õ������ԣ�
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ���
�ܣ���������������
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����N2�Ͼ�Cl2�����ռ����ܷ�
��ش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��2���ڢ۲����Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A�Ҷ�Ҫʹ������FeCl3�����ռ������ڢܲ������� ��
��3�����������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ� ��
��4��װ��D��FeCl2ȫ����Ӧ����ʧȥ����Cl2�����ö�ʧЧ��д������FeCl2�Ƿ�ʧЧ���Լ��� ��
��5�������߿��л���β������װ��E��ע���Լ���
����ͬѧ��װ��D�еĸ���ƷFeCl3��Һ����H2S���õ��������˺�����ʯīΪ�缫����һ�������µ����Һ��
��6��FeCl3��H2S��Ӧ�����ӷ���ʽΪ ��
��7��������H+�������ŵ����H2�������ĵ缫��ӦʽΪ ��
��8���ۺϷ���ʵ����������Ӧ����֪��ʵ�������������ŵ㣺
��H2S��ԭ��������Ϊ100%���� ��
��1��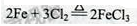
��2���ڳ����ĵ�FeCl3�����·����ȡ�
��3���ڡ��ݣ�
��4�����Ը��������Һ
��5��
��6��
��7��Fe2+-e-=Fe3+
��8��FeCl3����ѭ�����á� ��ÿ��2�֣�
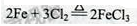
��2���ڳ����ĵ�FeCl3�����·����ȡ�
��3���ڡ��ݣ�
��4�����Ը��������Һ
��5��

��6��

��7��Fe2+-e-=Fe3+
��8��FeCl3����ѭ�����á� ��ÿ��2�֣�
�����������1��A�з�Ӧ�Ļ�ѧ����ʽ

��2��Ҫʹ������FeCl3�����ռ���������FeCl3���������������ʣ��ڢܵIJ���Ӧ���ǣ��ڳ����ĵ�FeCl3�����·����ȡ�
��3��Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�Т�ͨ������Cl2�����ø����N2�Ͼ�Cl2��
��4��װ��D��FeCl2ȫ����Ӧ�����Ϊʧȥ����Cl2�����ö�ʧЧ������FeCl2�Ƿ�ʧЧ���Ǽ�����������ӣ������
 ��Ҳ���������Ը��������Һ���顣
��Ҳ���������Ը��������Һ���顣��5��
 �����յ������������ÿ��Ƿ�����
�����յ������������ÿ��Ƿ�������6��

��7�������ĵ缫��Ӧ��

��8����ʵ�����һ���ŵ���FeCl3����ѭ�����á�

��ϰ��ϵ�д�
�����Ŀ