��Ŀ����
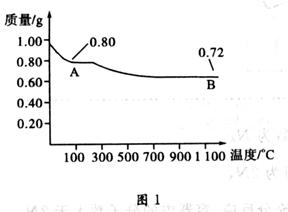
ͭ�����ֳ����������CuO��Cu2O��ijѧϰС��ȡ0.98 g Cu(OH)2 ������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ��

�����з�����ȷ���ǣ� ��
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������C
D��ͼ1�У�A��B��������0.01 mol���ӷ�����ת��


�����з�����ȷ���ǣ� ��
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������C
D��ͼ1�У�A��B��������0.01 mol���ӷ�����ת��
D
���������0.98gCu(OH)2��������Ϊ0.8g�����ķ�ӦΪCu(OH)2
 CuO��H2O,0.8gCuO��������Ϊ0.72g����ӦΪ4CuO
CuO��H2O,0.8gCuO��������Ϊ0.72g����ӦΪ4CuO 2Cu2O��O2��A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCuO��Cu2O������B������0.18gH2O������C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������B������ D����4CuO
2Cu2O��O2��A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCuO��Cu2O������B������0.18gH2O������C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������B������ D����4CuO 2Cu2O��O2����֪����ת����0.01 mol���ӣ���ȷ��
2Cu2O��O2����֪����ת����0.01 mol���ӣ���ȷ��
��ϰ��ϵ�д�
�����Ŀ