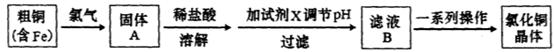��Ŀ����
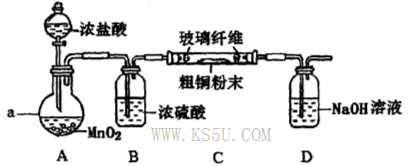
ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

������������̣��ش��������⣺
��1������I��������Һ�����õ��IJ����������ձ����������⣬�������� �� .�����������ƣ�
��2�����в�������ʹ������ҺŨ��ƫС����________________����д��ţ���
��δϴ���ձ��Ͳ�����
�ڶ���ʱ��������ƿ�Ŀ̶���
������Һǰ����ƿ������������ˮ
��ҡ�Ⱥ���Һ����ڿ̶��ߺ������ˮ����Һ����̶�������
��3����д��������ˮ���������ӷ���ʽ ��
��4����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ����� ��
��5����ԭ��Ʒ����aΪ50g�����Ⱥ����ɫ��������bΪ3g������Ʒ����Ԫ�ص����������� ��

������������̣��ش��������⣺
��1������I��������Һ�����õ��IJ����������ձ����������⣬�������� �� .�����������ƣ�
��2�����в�������ʹ������ҺŨ��ƫС����________________����д��ţ���
��δϴ���ձ��Ͳ�����
�ڶ���ʱ��������ƿ�Ŀ̶���
������Һǰ����ƿ������������ˮ
��ҡ�Ⱥ���Һ����ڿ̶��ߺ������ˮ����Һ����̶�������
��3����д��������ˮ���������ӷ���ʽ ��
��4����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ����� ��
��5����ԭ��Ʒ����aΪ50g�����Ⱥ����ɫ��������bΪ3g������Ʒ����Ԫ�ص����������� ��
��1��250mL����ƿ��1�֣�����ͷ�ιܣ�1�֣�
��2���٢� ��2�֣�
��3��2Fe 2+��Cl2��2Fe 3+�� 2Cl�� ��2�֣�
��4��ȡ����ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ� ��2�֣�
��5����Ԫ�ص�����������42%
��2���٢� ��2�֣�
��3��2Fe 2+��Cl2��2Fe 3+�� 2Cl�� ��2�֣�
��4��ȡ����ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ� ��2�֣�
��5����Ԫ�ص�����������42%
�����������1����ͼ��֪������I�ǽ��������ᷴӦ����Һϡ�ͳ�250.00mL��Һ������Ҫ250mL����ƿ����ͷ�ιܣ�
��2���٢�ƫС����ƫ�ߣ�����Ӱ��
��3������ˮ������+2������Ϊ+3�ۣ�������ӦΪ2Fe 2++Cl2=2Fe 3++2Cl-��
��4��ϴ�ӵ�������������������Һ�����Ȼ����Һ�����Լ���Cl���������Ƿ�ϴ�Ӹɾ���ȡ���һ��ϴ��Һ���μӵμ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��5����250ml��Һ��ȡ25ml��Һ����õ�����ɫ��������bΪ3g����ԭ����250ml��Һ�õ�����ɫ��������Ϊ30g,��������������������112/116������30g��������������112/116��30g. ��Ʒ����Ԫ�ص�����������112/116��30g/50g��100�G=42�G

��ϰ��ϵ�д�
�����Ŀ
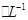 �������Һ������ܽ����۵�����Ϊ
�������Һ������ܽ����۵�����Ϊ 3Fe+4CO2������1.5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ����� ��
3Fe+4CO2������1.5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ����� ��