��Ŀ����
7����ҵ����ȡ����淋�����ͼ��ͼ1����ش��������⣺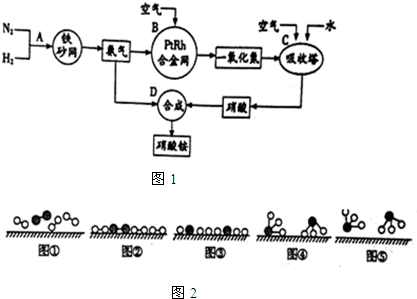
��l���ϳɰ��Ĺ�ҵ�豸�����Ǻϳ������豸�������Ƚ�������Ŀ�����������ȣ���Լ��Դ�������������У�N2��H2�ϳ�NH3���õĴ�����ɰ����������ԭ���������������Ŀ���Ƿ�ֹ�����ж���
��2��1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰���2007�껯ѧ�Ҹ����•���ֶ��ڹ����� ����֤ʵ�˰����뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ��ͼ��ͼ2��
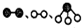 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���N2��H2�������ڴ������棻�ڴ�������N2��H2�еĻ�ѧ�����ѣ�
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���N2��H2�������ڴ������棻�ڴ�������N2��H2�еĻ�ѧ�����ѣ���3���������з�ӦΪ4NO+3O2+2H2O=4HNO3�����������̿�������������Ҫ�����������ԭ���ǿ�ʹNOѭ�����ã����ԭ�������ʣ�
��4����������Ĺ����г������һЩ��������������������ַ���������
����һ�������շ���NO+NO2+2NaOH=2NaNO2+H2O��2NO2+Na2CO3=NaNO2+NaNO3+CO2
��������NH3��ԭ����8NH3+6NO2$\frac{\underline{\;����\;}}{\;}$ 7N2+12H2O��NOҲ�����Ƶķ�Ӧ��
���������������շ���CH4��g��+2NO2��g��=CO2��g��+N2��g��+2H2O��g����H=+867kJ•mol-1 ��NOҲ�����Ƶķ�Ӧ��
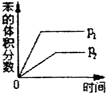
�������ַ����з���һ����ȱ���ǵ�����NO���ܱ����գ��������ͷ�������ȣ��ŵ��Ǽ���Ȱ��۸���ˣ�ȱ���Ǻ��ܸߣ�
��5��ij���ʳ���NH3�Ʊ�NH4NO3����֪����NH3��NO�IJ���94%��NO��HNO3�IJ�����89%������HNO3���õ�NH3������ռ�ܺ�NH3������������������ģ���54.4%��
���� ��1���ϳɰ��Ĺ�ҵ�豸�Ǻϳ������ϳɰ��ķ�Ӧ���ڷ��ȷ�Ӧ��N2��H2�ϳ�NH3���õĴ�������ɰ����������Ӧ��ֹ�����ж���
��2��ͼ��������˫ԭ�ӷ��ӱ������ڴ������棬��N2��H2�������ڴ������棻���з����еĻ�ѧ����������ԭ�ӣ����ڴ�������N2��H2�еĻ�ѧ����������Nԭ�Ӻ�Hԭ�ӣ�
��3�����������ж���������ˮ��Ӧ���������NO��ͨ�������NO�ܱ������е���������Ϊ����������������������ˮ��Ӧ�������ᣬ����ʹNOѭ�����ã�ȫ��ת��Ϊ���
��4������һ����ȱ���ǵ�����NO���ܱ����գ�ֻ����NO2һ�𱻼�Һ���գ��������ͷ�������ȣ��ŵ��Ǽ���Ȱ��۸���ˣ�ȱ���Ƿ������ĺ��ܽϸߣ�
��5�����ݵ�ԭ���غ��֪��NH3��NO��HNO3���Դ˼��㣮
��� �⣺��1���ϳɰ��Ĺ�ҵ�豸�Ǻϳ������ϳɰ��ķ�Ӧ���ڷ��ȷ�Ӧ����Ӧ�����л�ų��������ȣ����Ƚ��������Գ���������ȣ���Լ��Դ��N2��H2�ϳ�NH3���õĴ�������ɰ����������ԭ�����������������Է�ֹ�����ж���
�ʴ�Ϊ���ϳ������������ȣ���Լ��Դ����ɰ������ֹ�����ж���
��2��ͼ��������˫ԭ�ӷ��ӱ������ڴ������棬��N2��H2�������ڴ������棻���з����еĻ�ѧ����������ԭ�ӣ����ڴ�������N2��H2�еĻ�ѧ����������Nԭ�Ӻ�Hԭ�ӣ�
�ʴ�Ϊ��N2��H2�������ڴ������棻�ڴ�������N2��H2�еĻ�ѧ�����ѣ�
��3�����������ж���������ˮ��Ӧ���������NO��ͨ�������NO�ܱ������е���������Ϊ����������������������ˮ��Ӧ�������ᣬ����ʹNOѭ�����ã�ȫ��ת��Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ4NO+3O2+2H2O=4HNO3��
�ʴ�Ϊ��4NO+3O2+2H2O=4HNO3��ʹNOѭ�����ã�ȫ��ת��Ϊ���
��4������һ����ȱ���ǵ�����NO���ܱ����գ�ֻ����NO2һ�𱻼�Һ���գ��������ͷ�������ȣ��ŵ��Ǽ���Ȱ��۸���ˣ���Լ�ɱ���ȱ���Ƿ������ķ�Ӧ��Ϊ+867kJ•mol-1�����ܽϸߣ�
�ʴ�Ϊ��������NO���ܱ����գ�����Ȱ��۸���ˣ����ܸߣ�
��5����NH3��NO�IJ�����94%��NO��HNO3�IJ�����89%�����ݵ�ԭ���غ��֪��NH3��NO��HNO3����1mol�����ɵõ�����1mol��94%��89%=0.8366mol����HNO3+NH3�TNH4NO3����÷�Ӧ���ĵİ��������ʵ���Ϊ0.8366mol������������֮�ȵ������ʵ���֮�ȣ�����HNO3����ȥ��NH3������ռ�ܺ�NH3�����İٷ���Ϊ $\frac{1mol}{1mol+0.8366mol}$��100%=54.4%������HNO3����ȥ��NH3������ռ�ܺ�NH3������54.4%��
�ʴ�Ϊ��54.4��
���� ���⿼���˹�ҵ�Ʊ�ԭ��Ӧ�ã����̷�����ʵ���Ʊ����ʵķ����жϣ��������ʵ�ת���������غ�ķ��������м��㣬ʹ������淋�ע�������ȷת���еĻ�ѧ��Ӧ�ó�����֮��Ĺ�ϵ�ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | �ƺͼصĺϽ��ڳ�������Һ�壬�����ڿ����ӷ�Ӧ�����Ƚ����� | |
| B�� | �Ȼ�����Һ�����ԣ������������������Ȼ�����Һ | |
| C�� | ̼������Һ�ʼ��ԣ������ȵĴ�����Һ��ȥ���������� | |
| D�� | �����£���ҵ���ô����ʯӢɰ�Ʋ�����˵�����������ǿ��̼�� |
| A�� | 7��2 | B�� | 4��5 | C�� | 5��4 | D�� | 2��7 |
| A�� | ��ȥMgCl2��Һ�е�Fe3+��������Һ�м��백ˮ����pH | |
| B�� | ��ȥ��ˮ�еĽ�������Cu2+��Hg2+��������Һ�м���FeS | |
| C�� | ��ȥCl2�л��е�HCl�����Խ�����ͨ�뱥���Ȼ�����Һ | |
| D�� | ��ȥCaCO3�е�����CaSO4�����������м�����������Na2CO3��Һ����� |
| A�� | 1molij��������Ϊ22.4L���������״����һ���DZ�״�� | |
| B�� | 1 mol H2O�к��е�������Ϊ9NA | |
| C�� | 2.4gþ��ԭ�ӱ�Ϊ����ʱ��ʧ����Ϊ0.1NA | |
| D�� | ��1mol CO2�����ɹ��壬�������ķ�����С��NA |
| A�� |  | B�� | 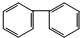 | C�� | 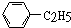 | D�� |  |
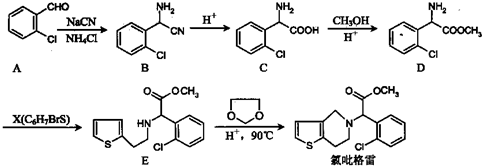
 ��
�� ��
��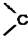 =O$��_{H+}^{ROH}$
=O$��_{H+}^{ROH}$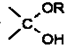 $��_{H+}^{ROH}$
$��_{H+}^{ROH}$ д������ϩ���״�Ϊ�л�ԭ���Ʊ�������
д������ϩ���״�Ϊ�л�ԭ���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2$\stackrel{Br_{2}}{��}$CH2Br•CH2Br��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2$\stackrel{Br_{2}}{��}$CH2Br•CH2Br��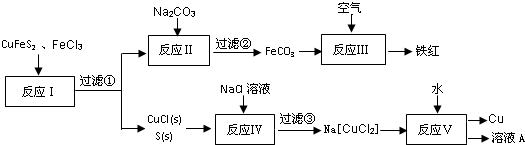
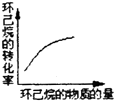
 ��g��$?_{����}^{Pt-Sn/Al_{2}O_{3}}$
��g��$?_{����}^{Pt-Sn/Al_{2}O_{3}}$ ��g��+3H2��g����H��0�����÷�Ӧ�ں��ݵ��ܱ������н��У������йظ÷�Ӧ��ͼ���ж���ȷ���ǣ�������
��g��+3H2��g����H��0�����÷�Ӧ�ں��ݵ��ܱ������н��У������йظ÷�Ӧ��ͼ���ж���ȷ���ǣ�������