��Ŀ����
16�����������ҹ����ֵ�����̷���һЩ�Է��Ȱ�ѪС�����Ϊ��Ҫ���ֵĸ�Ⱦ�Լ����������������ף�clopidogrel��1����һ����������ѪС��ۼ���ҩ�����ԭ�ϵIJ�ͬ����ҩ��ĺϳ�·��ͨ����������������2-�ȱ���ȩΪԭ�ϵĺϳ�·�����£�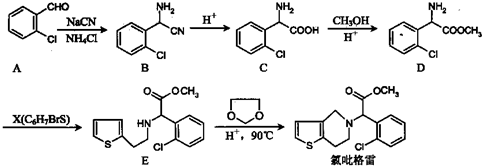
��1��X�Ľṹ��ʽΪ
 ��
����2������C����һ�������·�Ӧ����һ�ֲ���ò�������к���3����Ԫ����д���÷�Ӧ�Ļ�ѧ����ʽ
 ��
����3��C��D�ķ�Ӧ������������ȡ������Ӧ������D�����ֽṹ����ֻ��һ���ܺϳɾ���ҩ�����õ��������ף�����D�����ֽṹ��ԭ����D��������̼�����Խṹ��ֹһ�֣�
��4��д��A�����ڷ����廯���������ͬ���칹��Ľṹ��ʽ��
��5����֪��
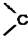 =O$��_{H+}^{ROH}$
=O$��_{H+}^{ROH}$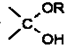 $��_{H+}^{ROH}$
$��_{H+}^{ROH}$ д������ϩ���״�Ϊ�л�ԭ���Ʊ�������
д������ϩ���״�Ϊ�л�ԭ���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2$\stackrel{Br_{2}}{��}$CH2Br•CH2Br��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2$\stackrel{Br_{2}}{��}$CH2Br•CH2Br��
���� ��1����D��E�Ľṹ��֪��D��E����ȡ����Ӧ����E�Ľṹ��֪X�Ľṹ��
��2��������C����һ�������·�Ӧ����һ�ֲ���ò�������к���3����Ԫ��������ȡ����Ӧ������-CO-NH-�ṹ��
��3��C��DΪ�����봼����������Ӧ������D�Ľṹ��ʽ��֪��D��������̼��
��4��A���ڷ����廯���������ͬ���칹���к��б������ɣ��ı�����Ż�����ŵ�λ����������
��5���Ʊ������� ����ϩ�ӳɺ�ˮ�������Ҷ������״�����������HCO������Ҷ������ȩ��Ӧ����
����ϩ�ӳɺ�ˮ�������Ҷ������״�����������HCO������Ҷ������ȩ��Ӧ���� ��
��
��� �⣺��1����D��E�Ľṹ��֪��D��E����ȡ����Ӧ����E�Ľṹ��֪X�ĽṹΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��������C����һ�������·�Ӧ����һ�ֲ���ò�������к���3����Ԫ��������ȡ����Ӧ������-CO-NH-�ṹ��
�÷�ӦΪ ��
��
�ʴ�Ϊ�� ��
��
��3��C��DΪ�����봼����������Ӧ������ȡ����Ӧ������D�Ľṹ��ʽ��֪��D��������̼�����Խṹ��ֹһ�֣�
�ʴ�Ϊ��������ȡ������D��������̼�����Խṹ��ֹһ�֣�
��4��A���ڷ����廯���������ͬ���칹���к��б������ɣ������������ͬ���칹����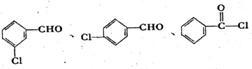 ��
��
�ʴ�Ϊ�� ��
��
��5���Ʊ������� ����ϩ�ӳɺ�ˮ�������Ҷ������״�����������HCO�������Ϣ��֪����Ҷ������ȩ��Ӧ����
����ϩ�ӳɺ�ˮ�������Ҷ������״�����������HCO�������Ϣ��֪����Ҷ������ȩ��Ӧ���� ���÷�Ӧ����Ϊ
���÷�Ӧ����Ϊ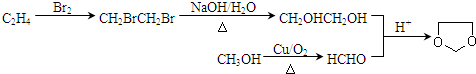 ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л���ĺϳɣ���ȷ�ϳ�·����̼���Ǽܵı仯�������Ļ�ѧ��Ӧ�Ƴ��������ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�| A�� | ú����������Һ�����������仯���ɱ�Ϊ�����Դ | |
| B�� | ����������ͺͿ�������Ҫ��ѧ�ɷ���ͬ | |
| C�� | ������顱��Ч�ɷ�Ϊ���ᣬ��Ư�ۻ��ʹ��Ч������ | |
| D�� | ������ʢ����ţ�̣�����ļ����������ӣ���ɱ��ţ���е�ϸ������ֹţ�̱��� |
| A�� | ��ʹpH��ֽ�ʺ�ɫ����Һ�п��ܴ��������������ӣ�Na+��NH4+��I-��NO3- | |
| B�� | ��CH3COOH��Һ��NaOH��Һ�������ϣ�����Һ��һ�����ڵĹ�ϵʽΪ��c��Na+��=c��CH3COO-��+c��CH3COOH�� | |
| C�� | pH=3��NaHA��Һ������Ũ�ȴ�СΪ��c��Na+����c��HA-����c��A2-����c��H2A����c��OH-�� | |
| D�� | CuSO4��Һ������п��ZnS������ת��Ϊͭ����CuS��������ΪZnS���ܽ�ȴ���CuS |
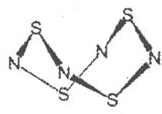
 A��B��C��D�ֱ��������ֶ�����Ԫ����ɵij������������ӣ������������о�������ͬ��Ŀ�ĵ��ӣ��ҹ���������Ԫ�أ�����A�к���5��ԭ�Ӻˣ�����֮�������µķ�Ӧ��ϵ��
A��B��C��D�ֱ��������ֶ�����Ԫ����ɵij������������ӣ������������о�������ͬ��Ŀ�ĵ��ӣ��ҹ���������Ԫ�أ�����A�к���5��ԭ�Ӻˣ�����֮�������µķ�Ӧ��ϵ�� ��D
��D ��
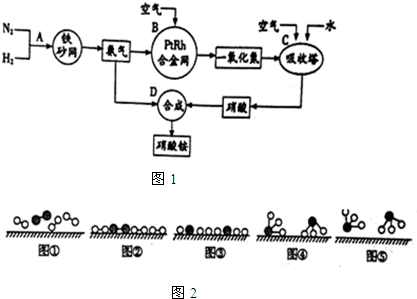
��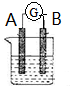 ��ͼ��ʾΪԭ���װ��ʾ��ͼ��
��ͼ��ʾΪԭ���װ��ʾ��ͼ��
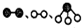 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���N2��H2�������ڴ������棻�ڴ�������N2��H2�еĻ�ѧ�����ѣ�
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���N2��H2�������ڴ������棻�ڴ�������N2��H2�еĻ�ѧ�����ѣ�