��Ŀ����
2������23V�����ҹ��ķ��Ԫ�أ��㷺���ڴ���������ҵ���ش��������⣺
��1������Ԫ�����ڱ��е�λ��Ϊ��4���ڢ�B�壬��۲�����Ų�ͼΪ
 ��
����2������ij��������ľ����ṹ��ͼ1��ʾ��������ʵ��ӵ�е����������Ӹ����ֱ�Ϊ4��2��
��3��V2O5������SO2ת��ΪSO3�Ĵ�����SO2������Sԭ�Ӽ۲���Ӷ�����3�ԣ����ӵ����幹��ΪV�Σ�SO3��̬Ϊ�����ӣ��÷�����Sԭ�ӵ��ӻ��������Ϊsp2��SO3�������廷״�ṹ��ͼ2��ʾ���ýṹ��Sԭ�ӵ��ӻ��������Ϊsp3���ýṹ��S-O���������࣬һ�����Լ140pm����һ�����Լ160pm���϶̵ļ�Ϊa����ͼ2����ĸ�����÷����к���12���Ҽ���

��4��V2O5�ܽ���NaOH��Һ�У��ɵõ������ƣ�Na3VO4�������������ӵ����幹��Ϊ�������壻Ҳ���Եõ�ƫ�����ƣ��������ӳ���ͼ3��ʾ��������״�ṹ����ƫ�����ƵĻ�ѧʽΪNaVO3��
���� ��1��������֪�����ĺ˵����Ϊ23���������֪����Ԫ�����ڱ��е�λ��Ϊ��4���ڢ�B�壬���ݺ�����ӵĹ�������Ų�˳��֪��1s��2s��2p��3s��3p��4s��3d��4p������ƶ�������Ų�ʽΪ1s22s22p63s23p63d34s2��ע������4s���������3d��������ͣ��������4s����������۲�����Ų�ʽΪ3d34s2���Դ���д�����Ų�ͼ��
��2���ɾ�����֪��Vλ�ڶ�������ģ�O��4��λ�����ģ�2��λ�����ģ�
��3��SO2������Sԭ���γ�2���Ҽ����µ��Ӷ���Ϊ$\frac{6-2��2}{2}$=1��SO3��̬Ϊ�����ӣ��÷�����Sԭ���γ�3���Ҽ���û�й¶Ե��ӣ�SO3����������Sԭ���γ�4���Ҽ����Դ��жϿռ乹�ͺ��ӻ����ͣ�SO3����������ÿ��S�γɣ�����S=O����S-O����S=O�����϶̣�
��4��VO43-�У�V�γ�4���Ҽ����µ��Ӷ���Ϊ$\frac{5+3-4��2}{2}$=0��Ϊ��������ṹ������״�ṹ��֪ÿ��V��3��O�γ������ӣ���V�Ļ��ϼ�Ϊ+5�ۣ��Դ��ж��γɵĻ�����Ļ�ѧʽ��
��� �⣺��1��������֪�����ĺ˵����Ϊ23���������֪����Ԫ�����ڱ��е�λ��Ϊ��4���ڢ�B�壬���ݺ�����ӵĹ�������Ų�˳��֪��1s��2s��2p��3s��3p��4s��3d��4p������ƶ�������Ų�ʽΪ1s22s22p63s23p63d34s2��ע������4s���������3d��������ͣ��������4s����������۲�����Ų�ʽΪ3d34s2��������Ų�ͼΪ ��
��
�ʴ�Ϊ����4���ڢ�B�壻 ��
��
��2���ɾ�����֪��Vλ�ڶ�������ģ������Ӹ���Ϊ1+8��$\frac{1}{8}$=2��O��4��λ�����ģ�2��λ�����ģ��������Ӹ���Ϊ4��$\frac{1}{2}$+2=4��
�ʴ�Ϊ��4��2��
��3��SO2������Sԭ���γ�2���Ҽ����µ��Ӷ���Ϊ$\frac{6-2��2}{2}$=1��SO2������Sԭ�Ӽ۲���Ӷ�����3��ΪV�νṹ��
SO3��̬Ϊ�����ӣ��÷�����Sԭ���γ�3���Ҽ���û�й¶Ե��ӣ���Ϊsp2�ӻ���
SO3����������Sԭ���γ�4���Ҽ���Ϊsp3�ӻ���SO3����������ÿ��S�γɣ�����S=O����S-O����S=O�����϶̣���a�϶̣��÷����к��ЦҼ���ĿΪ3��4=12��
�ʴ�Ϊ��3��V�Σ�sp2��sp3��a��12��
��4��VO43-�У�V�γ�4���Ҽ����µ��Ӷ���Ϊ$\frac{5+3-4��2}{2}$=0��Ϊ��������ṹ������״�ṹ��֪ÿ��V��3��O�γ������ӣ���V�Ļ��ϼ�Ϊ+5�ۣ����γɵĻ����ﻯѧʽΪNaVO3���ʴ�Ϊ���������壻NaVO3��
���� ����Ϊ2015�꺣��ѡ���⣬�ۺϿ������ʵĽṹ�����ʣ�������ѧ���ķ��������Ŀ��飬ע������ӻ������Լ��۲���������жϣ��Ѷ��еȣ�

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�| A�� | 1molCnH2n+2�к��е�C-C����Ϊ��n-1��NA | |
| B�� | 4.2 g C3H6�к��е�̼̼˫����һ��Ϊ0.1 NA | |
| C�� | 1 mol-OH�е�����Ϊ10 NA | |
| D�� | ��״���£�2.24 L CHCl3��ԭ������Ϊ0.5 NA |
| A�� | ����ˮ���������� | B�� | ʯ���ѽ��Ʊ�ϩ | ||
| C�� | �Ҵ������ᷴӦ���������� | D�� | ��֬��ŨNaOH��Ӧ�Ƹ�֬������ |
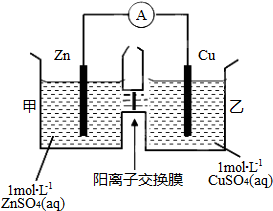
| A�� | ͭ�缫�Ϸ���������Ӧ | |
| B�� | ��ع���һ��ʱ��׳ص�c��SO42-����С | |
| C�� | ��ع���һ��ʱ����ҳ���Һ������������ | |
| D�� | �������ӷֱ�ͨ������Ĥ���������ƶ���������Һ�е��ƽ�� |
| A�� | ������Ϊ17��������Ϊ20����ԭ��${\;}_{17}^{20}$Cl | |
| B�� | �����ӣ�Cl-���Ľṹʾ��ͼ��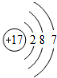 | |
| C�� | �ȷ��ӵĵ���ʽ��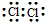 | |
| D�� | ����ϩ���ӵĽṹ��ʽ��H3C-CH2Cl |
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ����ɫ��Һ$\stackrel{NaOH��Һ}{��}$���ɫ���� | ˵��ԭ��Һ��һ��������FeCl3 |
| B | CaO$\stackrel{H_{2}O}{��}$Ca��OH��2$\stackrel{Na_{2}CO_{3}}{��}$NaOH | ����ʯ���Ʊ�NaOH��Һ |
| C | ���ռ�������$\stackrel{Ba��NO_{3}��_{2}��Һ}{��}$��ɫ���� | ������һ������SO42- |
| D | H3PO3+2NaOH��������-Na2HPO3+2H2O | H3PO3������Ԫ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |

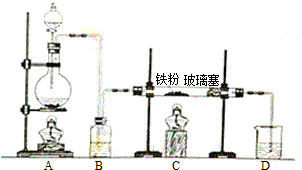
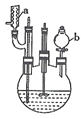 �Ѷ�����һ����Ҫ���л���Ԫ�ᣬ��Ҫ������������66��ά������66��֬����ʵ�����п�ͨ����������������Ӧ���Ʊ�����Ӧԭ����3
�Ѷ�����һ����Ҫ���л���Ԫ�ᣬ��Ҫ������������66��ά������66��֬����ʵ�����п�ͨ����������������Ӧ���Ʊ�����Ӧԭ����3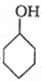 +8HNO3$\stackrel{һ������}{��}$3HOOC��CH2��4COOH+8NO��+7H2O����Ӧװ�ã����ּг�װ�ú���Դ��ʡ�ԣ���ͼ��ʾ��
+8HNO3$\stackrel{һ������}{��}$3HOOC��CH2��4COOH+8NO��+7H2O����Ӧװ�ã����ּг�װ�ú���Դ��ʡ�ԣ���ͼ��ʾ��