��Ŀ����
12�������ǹ�ҵ��������Ҫ�IJ�Ʒ֮һ���Ի�����Ϊԭ����������ļ�������ͼ��ʾ��
����д���пհף�
��1��������ȼ������SO2�Ļ�ѧ����ʽΪ4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��2��Ϊ������÷�Ӧ�ų����������Ӵ�����Ӧ��װ�Ƚ����������豸���ƣ��������������������ɹܣ�������Ϊ�������������Ũ����ĽӴ�����������ڶ�����������գ�
��3��Ϊʹ���������ճ�֣���ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ0.11����������������������Ϊ0.20����������¯��������������ֱ������Ӵ��ң�����Ϊ1.00m3•s-1���ӽӴ��ҵ������������Ϊ0.95m3•s-1��ͬ��ͬѹ�²ⶨ������SO2��ת����Ϊ92.5%��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Σ�SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽΪSO2+Br2+2H2O�T4H++2Br-+SO42-��
��5���������;�dz��㷺����Ӧ����bcd���ɶ�ѡ����
a������ b��������Ƶ��Ʊ�
c��Ǧ���ص����� d��������Լ�������������ơ��ĺϳɣ�
���� ��1��������ȼ������SO2�������������������ԭ�ӡ������غ���д����ʽ��
��2��������÷�Ӧ�ų�����������Ҫ�Ƚ������������������������ɹܿ���������ʱ�Ӵ������
��3��������Ӧ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��������8molSO2����μӷ�Ӧ������11mol����������ʵ��ͨ���O2���ʵ������ټ�������е��������ʵ�����ʣ���������ʵ������������������������������
�Ӵ����з�����Ӧ��O2+2SO2$\frac{\underline{����}}{��}$2SO3�������Ӵ��ҵ��������Ϊ1L����������ϵ��֪���ӽӴ��ҵ�������Ϊ0.95L����������������������ò���������μӷ�Ӧ������������������������������ת���ʣ�
��4��SO2����Br2��Ӧ���������HBr��
��5��������������ʵ��Ʊ����ϳɣ�Ҳ��������ʣ�
��� �⣺��1��������ȼ������SO2������������������ԭ�ӡ������غ��֪����ʽΪ4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2���ʴ�Ϊ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��2��Ϊ������÷�Ӧ�ų����������Ӵ�����Ӧ��װ�Ƚ������������������������ɹܣ�������Ϊ�������������Ũ����ĽӴ�����������ڶ�����������գ�
�ʴ�Ϊ���Ƚ��������������������Ũ����ĽӴ�����������ڶ�����������գ�
��3��������Ӧ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��������8molSO2����μӷ�Ӧ������11mol��ʵ��ͨ���O2Ϊ��1+40%����11mol=15.4mol�����Կ����е���Ϊ4��15.4mol=61.6mol��ʣ������Ϊ15.4mol-11mol=4.4mol�������պ�¯����SO2���������Ϊ$\frac{8mol}{8mol+4.4mol+61.6mol}$=0.11��
�Ӵ����з�����ӦO2+2SO2$\frac{\underline{����}}{��}$2SO3�������Ӵ��ҵ��������Ϊ1L����������ϵ��֪���ӽӴ��ҵ�������Ϊ0.95L�����������С��=1L-0.95L=0.05L���ɷ���ʽ��֪��2�����������Ӧ�������������С1������ʲμӷ�Ӧ�Ķ�������Ϊ0.05L��2=0.1L������Ӵ��ҵ���������SO2�Ļ�Ϊ1L��$\frac{8mol}{8mol+4.4mol+61.6mol}$=0.108L���ʶ��������ת����Ϊ$\frac{0.1L}{0.108L}$��100%=92.5%��
�ʴ�Ϊ��0.11��92.5%��
��4��SO2����Br2�����ӷ���ʽΪSO2+Br2+2H2O�T4H++2Br-+SO42-���ʴ�Ϊ��SO2+Br2+2H2O�T4H++2Br-+SO42-��
��5��a���������õ���Ϊ�����ʴ���
b��������Ƶ���ȡ��������ҪŨ�������ʯ������ȷ��
c��Ǧ��������Ҫ�õ����������Ǧ������ȷ��
d��������������к��л��������ȡ��������Ҫ�����ǻ���Ӧ������ȷ��
�ʴ�Ϊ��bcd��
���� ���⿼�����Ṥҵ��������Ʊ���Ϊ2015��߿��⣬�����Ʊ�Ũ����Ĺ������̼��豸�����á������Ļ�ѧ��ӦΪ���Ĺؼ�����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�
| A�� | PX�����мȺ��Ҽ��ֺ��м� | |
| B�� | PTA�Ǹ÷�Ӧ���������� | |
| C�� | PTA���Ҷ���ͨ���Ӿ۷�Ӧ��������PET���� | |
| D�� | �÷�Ӧÿ����1molPX����ת��12NA�����ӣ�NAΪ����٤�������� |
| A�� | 0.1mol•L-1 HA��Һ��0.1 mol•L-1��NaOH��Һ�У�ˮ���������c��H+����� | |
| B�� | ��Ϻ���Һ�У�c��HA����c��Na+����c��A-����c��OH-�� | |
| C�� | ��Ϻ���Һ�У�c��A-��+c��HA��=0.2 mol•L-1 | |
| D�� | ��Ϻ���Һ�У�c��Na+��+c��H+��=c��A-��+c��OH-�� |
 ����һ�ָ�Ч�����������к�ǿ�������Ժ�ʴ�ԣ������ɱ����������������һ���������Ƶã�������Ѹ��ɱ���������������ֲ�����ϸ��������������йع�������������в���ȷ���ǣ�������
����һ�ָ�Ч�����������к�ǿ�������Ժ�ʴ�ԣ������ɱ����������������һ���������Ƶã�������Ѹ��ɱ���������������ֲ�����ϸ��������������йع�������������в���ȷ���ǣ�������| A�� | ����������Һ��̼��Ʒ�Ӧ�ܲ���������̼���� | |
| B�� | �����������Ҵ��ܷ���������Ӧ | |
| C�� | �����������ǻ����ᣨHOCH2COOH����Ϊͬ���칹�� | |
| D�� | �ɹ��������������ȡ��������ķ�Ӧ����������ԭ��Ӧ |
| A�� | C99H200 | B�� | CO | C�� | NaHCO3 | D�� | CH3COOCH2CH3 |
 �ĺϳ�·����ͼ��ʾ��
�ĺϳ�·����ͼ��ʾ��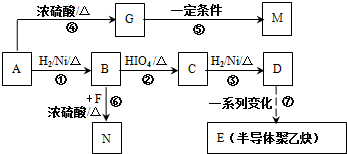
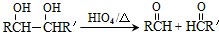
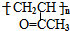 ��
�� ��
��
 ��
��