��Ŀ����
14���л���ۺ���M��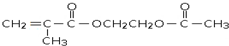 ��﮵��������֮�������Ǩ�ƵĽ��ʣ�����C4H8�ϳ�M�ĺϳ�·����ͼ��
��﮵��������֮�������Ǩ�ƵĽ��ʣ�����C4H8�ϳ�M�ĺϳ�·����ͼ��
��1��C4H8�Ľṹ��ʽΪ
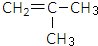 ���Լ�II��NaOHˮ��Һ��
���Լ�II��NaOHˮ��Һ����2������B��Ӧ������C�ķ�����ȡ������������Cu��OH��2����Һ���������У�����ש��ɫ������֤������C����ȡ��������������Һ��ˮԡ���ȣ�����������֤������C��
��3��D��һ���������ܷ������۷�Ӧ���ɸ߷��ӻ������Ӧ�Ļ�ѧ����ʽΪ
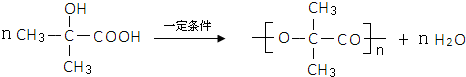 ��
����4����Ӧ����ۢܢݲ�����Ϊ�ݢۢܵ���Ҫ�����DZ���̼̼˫������������
���� ��ת����ϵ��֪C4H8��A��B��C��D��E��̼�Ǽܽṹ���䣬���M�뻷������ṹ��֪��F�����ᷢ��������Ӧ��M����Ҫ���Ƶ�FΪCH2=C��CH3��COOCH2CH2OH��EΪCH2=C��CH3��COOH�����C4H8��E�Ĺ����ű仯��֪���������Ʒ���֪��D��Ũ���������·�����ȥ��Ӧ����E����DΪHOOCC��OH����CH3��2��C��������D����CΪOHCC��OH����CH3��2��B����������C����BΪHOCH2C��OH����CH3��2��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B������AΪ ��C4H8���巢���ӳɷ�Ӧ����A������C4H8�Ľṹ��ʽΪ
��C4H8���巢���ӳɷ�Ӧ����A������C4H8�Ľṹ��ʽΪ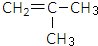 ���ݴ˽��
���ݴ˽��
��� �⣺��ת����ϵ��֪C4H8��A��B��C��D��E��̼�Ǽܽṹ���䣬���M�뻷������ṹ��֪��F�����ᷢ��������Ӧ��M����Ҫ���Ƶ�FΪCH2=C��CH3��COOCH2CH2OH��EΪCH2=C��CH3��COOH�����C4H8��E�Ĺ����ű仯��֪���������Ʒ���֪��D��Ũ���������·�����ȥ��Ӧ����E����DΪHOOCC��OH����CH3��2��C��������D����CΪOHCC��OH����CH3��2��B����������C����BΪHOCH2C��OH����CH3��2��A����������ˮ��Һ�����������·���ˮ�ⷴӦ����B������AΪ ��C4H8���巢���ӳɷ�Ӧ����A������C4H8�Ľṹ��ʽΪ
��C4H8���巢���ӳɷ�Ӧ����A������C4H8�Ľṹ��ʽΪ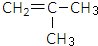 ��
��
��1����������ķ�����֪��C4H8�Ľṹ��ʽΪ 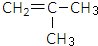 ���Լ�II��NaOHˮ��Һ��
���Լ�II��NaOHˮ��Һ��
�ʴ�Ϊ��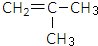 ��NaOHˮ��Һ��
��NaOHˮ��Һ��
��2��CΪOHCC��OH����CH3��2��BΪHOCH2C��OH����CH3��2��C����ȩ������B��û�У����Լ���B��Ӧ������C�ķ����ǣ�ȡ������������Cu��OH��2����Һ���������У�����ש��ɫ������֤������C����ȡ��������������Һ��ˮԡ���ȣ�����������֤������C��
�ʴ�Ϊ��ȡ������������Cu��OH��2����Һ���������У�����ש��ɫ������֤������C����ȡ��������������Һ��ˮԡ���ȣ�����������֤������C������������
��3��DΪHOOCC��OH����CH3��2��D��һ���������ܷ������۷�Ӧ���ɸ߷��ӻ������Ӧ�Ļ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
��4��̼̼˫���ܱ�����������������̼̼˫���γ�֮ǰ�Ƚ���������Ӧ�����Է�Ӧ����ۢܢݲ�����Ϊ�ݢۢܵ���Ҫ�����DZ���̼̼˫������������
�ʴ�Ϊ������̼̼˫������������
���� ���⿼���л�����ƶϣ��Ѷ��еȣ������������Ʒ����Ͻ����ƶϣ��Ƕ��л���ѧ֪ʶ���ۺ����ã�ע����M�Ľṹ�ж�F��E�Ľṹ�ǹؼ����ܽϺõĿ���ѧ���ķ�����˼ά������

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�| A�� | ��ϩ���Ҵ��� | B�� | ˳����ʯ���ѽ����� | ||
| C�� | �⣨������ | D�� | �壨��ˮɹ�κ����Һ�� |
| A�� | �����ͺ�ֲ���Ͷ�����ͨ��ʯ�ͷ�������ȡ | |
| B�� | ��������������Ѫ����ɳɷ� | |
| C�� | �����ʵ�ˮ����ﶼ�Ǧ�-������ | |
| D�� | ���PM2.5��ֱ��ԭ��������ɳĮ�� |
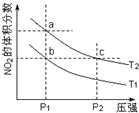 ��Ӧ2NO2��g��?N2O4��g��+57kJ���������������������䣮���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǣ�������
��Ӧ2NO2��g��?N2O4��g��+57kJ���������������������䣮���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | a��c�����������ɫ��adz��c�� | |
| B�� | a��c���������ƽ����Է���������a��c | |
| C�� | b��c�����ƽ�ⳣ����Kb=Kc | |
| D�� | ״̬aͨ�������¶ȿɱ��״̬b |
 ��֪ij������ľ�������������С��Ԫ���öѻ����ɵģ����ڸû������������������ȷ���ǣ�������
��֪ij������ľ�������������С��Ԫ���öѻ����ɵģ����ڸû������������������ȷ���ǣ�������| A�� | �û�����Ļ�ѧʽ��Y4Ba4Cu3O12 | B�� | �û�����Ļ�ѧʽ��YBaCu3O6 | ||
| C�� | �û�����Ļ�ѧʽ��Y2BaCu3O6 | D�� | �û�����Ļ�ѧʽ��YBa2Cu3O7 |
| A�� | N2��g��+3H2��g��?2NH3��g����H=-92.4kJ | |
| B�� | 2H2��g��+O2��g���T2H2O��l����H=+571.6KJ•mol-1 | |
| C�� | N2H4��g��+O2��g���TN2��g��+2H2O��g����H=-534.4kJ•mol-1 | |
| D�� | CH4+2O2�TCO2+2H2O��H=-890.3kJ•mol-1 |
| A�� | ͬ����Ԫ�ص�ԭ�Ӱ뾶�Ԣ�A���Ϊ��С | |
| B�� | �����ڱ�������Ԫ�صĵ���ȫ�������� | |
| C�� | ��A����A��Ԫ�ص�ԭ�ӣ���뾶Խ��Խ����ʧȥ���� | |
| D�� | ��������Ԫ�ص�ԭ���γɵ�ԭ������ʱ�������������������������� |
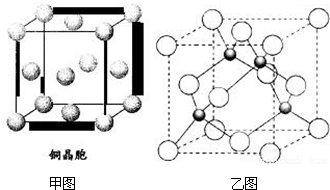
 ����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ�������3����EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���ش��������⣺
����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ�������3����EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���ش��������⣺ ��
��