��Ŀ����
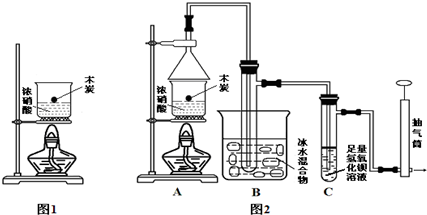
19��ijѧϰС������ͼ1װ����ȡ����������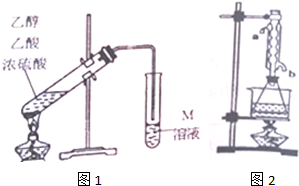
| ��Է������� | �е㣨�棩 | |
| �Ҵ� | 46 | 78.5 |
| ���� | 60 | 117.9 |
| �������� | 88 | 77.1 |
��1����ͼ1��ȡװ����Ҫ�������Ƭ���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������C������ȷѡ����ĸ����
A���������� B�������Ӳ� C����ȴ��Ӳ� D������Ӳ�
��2��M��ҺΪNa2CO3���ѧʽ����Һ��װ����ͨ�����ĵ���Ҫ���ڱ���M��Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹM��Һ��������ɵ�����ԭ����������Ҵ���������Na2CO3��Һ��
��3����ͬѧ��Ϊ�Ľ�ͼ1����ȡװ������������������ʣ�д��һ���Ľ����飺��װˮ�����ܣ������ȴЧ������Բ����ƿ��������ƿ������װ�¶ȼƿ��Ʒ�Ӧ�¶ȣ�
��4��ͼ1װ����ȡ�����������л��������Ҵ������ᣮͨ������ʵ��ɷ������������ĺ����������������£�
��ȷ����20.0g���������ֲ�Ʒ����ƿ�У���0.50mol•L-1NaOH�ζ�����̪��ָʾ�������յ�ʱ����NaOH��Һ�����Ϊ40.0mL
����ȡ20.0g���������ֲ�Ʒ��250mL��ƿ�У�����100mL2.1mol•L-1NaOH��Һ��Ͼ��Ⱥ�װ�������ܣ���ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ2��ʾ��������ȴ����0.50mol•L-1HCl�ζ�������NaOH���յ�ʱ������������Ϊ20.0mL��ʵ�������ˮ�������ܵ�b��ѡ��a��b���ܿ�ͨ�룮����ʵ��١��ڲ��������ݼ���ֲ�Ʒ��������������������Ϊ79.2%��
���� ��1�����Ƭ�Ĵ��ڿ��Է�ֹ�ڼ��ȹ����в����������������Ƭʱ��Ҫ���Ѽ��ȵ���Һ��ȴ���ټ��룻
��2���Թ���M��Һ������������������ͨ��ʹ��̼������Һ�������Ҵ�������������̼������Һ�����Ե��ܲ�����Һ��������������
��3�����Թܸij�Բ����ƿ��Ȼ���װ�¶ȼƿ����¶ȣ�������������IJ��ʣ�
��4��������������������ͨˮЧ������ɣ���������ʵ��١��ڲ��������ݼ���Ӧԭ��������ֲ�������������������������
��� �⣺��1��ȱ�����Ƭ�ᷢ�����������������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ô���Һ��ȴ���ټ��룬����C��ȷ��
�ʴ�Ϊ��C��
��2������������̼������Һ�е��ܽ�Ƚ�С����̼������Һ�ܹ��������ᡢ�ܽ��Ҵ�������MΪNa2CO3������������Ҵ���������Na2CO3��Һ�����Թ�����������������
�ʴ�Ϊ��Na2CO3��������Ҵ���������Na2CO3��Һ��
��3����������������ȡ�����У��Ҵ��������ܹ��ӷ�������Ӱ�������������IJ��ʣ����Լ�װˮ�����ܣ������ȴЧ����Ҳ������Բ����ƿ����Թܲ���װ�¶ȼƿ��Ʒ�Ӧ�¶ȵķ�����ͨ�����������IJ��ʣ�
�ʴ�Ϊ����װˮ�����ܣ������ȴЧ������Բ����ƿ��������ƿ������װ�¶ȼƿ��Ʒ�Ӧ�¶ȣ�����������Ҳ���֣���
��4����������������ͨˮ��ʹˮ�������������ֽӴ�������Ч���ã��ʴ�bͨˮ��
�ɢٿ�֪�����������Ʒ�Ӧ�������������е��������ᣬ����20g����������Ʒ�к�����������ʵ���Ϊ��0.50mol•L-1��0.04L=0.02mol��
���ݢڿ�֪����������ˮ�����ɵ�����������������ĵ��������Ƶ����ʵ���Ϊ��2.1mol•L-1��0.1L-0.50mol•L-1��0.02L=0.20mol��
��������ˮ�����ɵ���������ʵ���Ϊ��0.20mol-0.02mol=0.18mol��
�������������ʵ���������������ʵ���������20g����������Ʒ�к�����������������Ϊ��88g/mol��0.18g=15.84g��
��������������������$\frac{15.84g}{20g}$��100%=79.2%��
�ʴ�Ϊ��b��79.2%��
���� ���⿼����������������ȡ����Ŀ�Ѷ��еȣ�ע���������������ķ�Ӧԭ����װ��ѡ��������ע�ض�ѧ������֪ʶѵ���ͼ����ͬʱ�����ض�ѧ��ʵ����������������ͷ����뼼�ɵ�ָ����ѵ�������������ѧ����ʵ�����������Ӧ������������ѧ����ѧ��������

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�| A�� | SiO2+4HF=SiF4��+2H2O���������ʴ�̲��� | |
| B�� | 4Na+TiCl4 $\frac{\underline{\;����\;}}{\;}$4NaCl+Ti���ý�����������TiCl4��Ӧ��ȡ����Ti | |
| C�� | CO32-+H2O?H2CO3+OH-�����ȵĴ�����Һ��ϴ�;����� | |
| D�� | MgCl2$\frac{\underline{\;���\;}}{\;}$Mg+Cl2������ҵ���õ�������Ȼ�þ��ȡ����þ |
| A�� | 84 g NaHCO3�������NA��CO32- | |
| B�� | �����£�14.0 g��ϩ�Ͷ�ϩ�Ļ�������к���C-H����ĿΪ2.0NA | |
| C�� | ��⾫��ͭʱ����������������6.4 g�����·��ת�Ƶ�����Ϊ0.2NA | |
| D�� | 78 g Na2O2��������CO2��Ӧ������ת�Ƹ���Ϊ2NA |
����ƾ��壨CaC2O4•H2O������������ϡ�н��������壮
��1����һ���¶�����1L���ܱ�����������������ƣ�������ռ������Բ��ƣ�������Ӧ��CaC2O4��s��?CaO��s��+CO��g��+CO2��g������ǰ5min������CaO������Ϊ11.2g����ö�ʱ����v��CO��=0.04mol•L-1•min-1������Ӧ�ﵽƽ����������ѹ��Ϊ0.5L��һ��ʱ���Ӧ�ٴδﵽƽ�⣬������˵����ȷ����CD�����ѡ���ţ���
A��ѹ�����������CaC2O4�ķֽ�������
B��ƽ���ƶ��÷�Ӧ�Ļ�ѧƽ�ⳣ����С
C��ƽ���ƶ���CO��CO2��Ũ�ȶ�û�иı�
D��ƽ���ƶ���CaO����������
��2��ij�¶������ݣ�K����CaC2O4��=5.0��10-9��K����CaCO3��=2.5��10-9
��0.6mol/L��Na2CO3��Һ�м�������CaC2O4��ĩ������Һ����仯������ֽ��裬������Ӧ��
CO32-��aq��+CaC2O4��s��?CaCO3��s��+C2O42-��aq�������ú����ת���ﵽƽ�⣮���ʱ��Һ�е�c��C2O42-��=0.4mol•L-1��
����ˮ���������ƺϳ��������ü�����Դ��;��֮һ���ù��̵���Ҫ��Ӧ��CH4��g��+H2O��g��?CO��g��+3H2��g����H��0����������ͬʱ������ͬʱ���ڣ���Ӧ�ֱ��ڲ�ͬ��������ͬ�¶��·�Ӧ�����CH4��ת���ʱ仯��ͼ��ʾ��

��1������ͬ�����£����ִ�����Ĵ�Ч���ɸߵ��͵�˳���Ǣ�
��2��a��v����CH4������v����CO������д�����ڡ�����С�ڡ����ڡ�����
��3��c��CH4��ת���ʸ���b�㣬ԭ����b��c��ûƽ�⣬c���¶ȸߣ���Ӧ���ʿ죬��ͬʱ����ת���ʸߣ�
��ҵ���ý�̿��ˮ�����ڸ�������ȡˮú�����ڻ�������������Ҫ��;��һ�������£���C��s����H2O��g���ֱ����ס��������ܱ������У�������Ӧ��
C��s��+2H2O��g��?CO2��g��+2H2��g������������������ʾ��
| �ݻ� | �ݻ�/L | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | |
| C��s�� | H2O | H2 | ||||
| �� | 2 | T1 | 2 | 4 | 3.2 | 8 |
| �� | 1 | T2 | 1 | 2 | 1.2 | 3 |
��2���÷�Ӧ�ﵽƽ��������¶ȣ�H2O��g����ת���ʱ�С����д���������С������
| A�� | H2SO4+2NaOH�TNa2SO4+2H2O | B�� | CuO+H2 $\frac{\underline{\;\;��\;\;}}{\;}$ Cu+H2O | ||
| C�� | NH3+HCl�TNH4Cl | D�� | 2NaHCO3 $\frac{\underline{\;\;��\;\;}}{\;}$ Na2CO3+H2O+CO2�� |
| ��ѧʽ | ���볣�� |
| CH3COOH | K=1.7��10-5 |
| HCN | K=4.9��10-10 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
| A�� | H2CO3��HCO3-��CH3COO-��CN- | B�� | HCO3-��CH3COOH��CN-��CO32- | ||
| C�� | HCN��HCO3-��CN-��CO32- | D�� | HCN��HCO3-��CH3COO-��CN- |
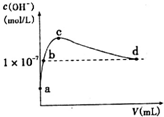 �����£���l L pH=l0��NaOH��Һ�г���ͨ��CO2��ͨ��CO2�������y������Һ��ˮ�������c��OH-���Ĺ�ϵ��ͼ��ʾ����������������ǣ�������
�����£���l L pH=l0��NaOH��Һ�г���ͨ��CO2��ͨ��CO2�������y������Һ��ˮ�������c��OH-���Ĺ�ϵ��ͼ��ʾ����������������ǣ�������| A�� | a����Һ�У�ˮ�������c��H+��=1��10-10mol•L-1 | |
| B�� | b����Һ�У�c��H+��=1��10-7mol•L-1 | |
| C�� | c����Һ�У�c��Na+����c��HCO3-����c��CO32-�� | |
| D�� | d����Һ�У�c��Na+��=2c��CO32-��+c��HCO3-�� |