��Ŀ����
7���͵����������岻��ȱ�ٵ�Ԫ�أ���������Ȼ�������������ε���ʽ���֣�������ƿ�Ca3��PO4��2����ʯCa5F��PO4��3�ȣ���1������23��ͬλ�أ���������������������ȵ�ͬλ���������õĵ�һ���˹�������ͬλ�أ��ú��ؿɱ�ʾΪ3015P��
��2���������ж���ͬ�������壬����ס����ס����ȣ�
��������뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1400-1500�����ɰ��ף���Ӧʽ��
2Ca3��PO4��2+6SiO2=6CaSiO3+P4O10��10C+P4O10=P4��+10CO��
������Ӧ�ĸ��������У�������������SiO2��P4O10���ѧʽ��������ϵһ���ڻ�ԭ�Ե������У���ôÿ����1mol P4ʱ��20mol���ӷ���ת�ƣ�
�ڻ��ף�P4���ж������ܽ�������ͭ�����������Һ�л�ԭ������д��������������ͭ��Һ��Ӧ�Ļ�ѧ����ʽP4+10CuSO4+16H2O=10Cu+4H3PO4+10H2SO4��
��3���Ƚ�N��P��̬�⻯����ȶ��ԣ�PH3��NH3�������������=����������ԭ�ӽṹ�Ĺ۵������ԭ��NPͬ����������������ͬ��P�ĵ��Ӳ�����N�࣬ԭ�Ӻ˶Ե��ӵ�������������������ȶ��Խϲ
��4����������ˮ��Һ�м��е�������ˮ��
�������Ƶ�ˮ��Һ�Լ��ԣ������ӷ���ʽ����ԭ��PO43-+H2O?HPO42-+OH-�����ж����Ӧ����д����Ҫ��Ӧ���ɣ���
����������Ԫ�ᣬ��298Kʱ��K1=7.5��10-3��K2=6.2��10-8��K3=2.2��10-13����NaH2PO4��Һ�����ԣ���ᡱ�����ͨ������˵���������Kh��H2PO4-��=$\frac{Kw}{{K}_{1}}$=$\frac{1��1{0}^{-14}}{7.5��1{0}^{-3}}$=1.33��10-10��K2��H2PO4-�ĵ���ǿ��ˮ�⣬��Һ��c��H+����c��OH-����
���� ��1������������������ȵ�ͬλ�أ�����õ�������=15+15=30�����ԭ�ӷ�����д���أ�
��2�����������������ܺͼӦ�����κ�ˮ��������ǽ����������зǽ������ϼۺ�����Ԫ�ػ��ϼ���ͬ�����������ԭ��Ӧ�����غ�������ת�ƣ�
�ڻ��ף�P4���ж������ܽ�������ͭ�����������Һ�л�ԭ������д��������������ͭ��Һ��Ӧ����ͭ����������ᣬ���ԭ���غ���ƽ��д��ѧ����ʽ��
��3������ͬ����ǽ����Ե�ǿ�������ж��⻯����ȶ��ԣ�ʵ����ԭ�ӽṹ������������ԭ�Ӻ˶��������ӵ��������жϣ�
��4���������Ƶ�ˮ��Һ�Լ�������Ϊ��������ӷֲ�ˮ�⣬��Һ��ˮ�ĵ��뱻�ٽ�����������Ũ������
��K1=7.5��10-3��K2=6.2��10-8��K3=2.2��10-13����NaH2PO4��Һ�з�������H2PO4-��ˮ�ⳣ������H2PO4-�ĵ���̶ȱȽϷ����ж���Һ����ԣ�
��� �⣺��1������������������ȵ�ͬλ�أ�����õ�������=15+15=30�����ԭ�ӷ�����д����Ϊ��3015P��
�ʴ�Ϊ��3015P��
��2�����������������ܺͼӦ�����κ�ˮ��������ǽ����������зǽ������ϼۺ�����Ԫ�ػ��ϼ���ͬ����Ӧ2Ca3��PO4��2+6SiO2=6CaSiO3+P4O10��10C+P4O10=P4��+10CO���У����������ǹ����������P4O10����������������������ԭ��Ӧ�����غ�������ת�ƣ�ÿ����1mol P4ʱ̼Ԫ�ػ��ϼ�0�۱仯Ϊ+2�ۣ���Ԫ�ػ��ϼ۱仯Ϊ+5�ۣ�����ת��Ϊ20mol��
�ʴ�Ϊ��20��
�ڻ��ף�P4���ж������ܽ�������ͭ�����������Һ�л�ԭ������д��������������ͭ��Һ��Ӧ����ͭ����������ᣬ���ԭ���غ���ƽ��д��ѧ����ʽΪ��P4+10CuSO4+16H2O=10Cu+4H3PO4+10H2SO4��
�ʴ�Ϊ��SiO2��P4O10��P4+10CuSO4+16H2O=10Cu+4H3PO4+10H2SO4��
��3������ͬ����ǽ����Ե�ǿ�������ж��⻯����ȶ��ԣ�ʵ����ԭ�ӽṹ������������ԭ�Ӻ˶��������ӵ��������жϣ�N��Pͬ����������������ͬ��P�ĵ��Ӳ�����N�࣬ԭ�Ӻ˶Ե��ӵ�������������������ȶ��ԽϲPH3��NH3��
�ʴ�Ϊ������N��Pͬ����������������ͬ��P�ĵ��Ӳ�����N�࣬ԭ�Ӻ˶Ե��ӵ�������������������ȶ��Խϲ
��4���������Ƶ�ˮ��Һ�Լ�������Ϊ��������ӷֲ�ˮ�⣬��Һ��ˮ�ĵ��뱻�ٽ�����������Ũ������ˮ�����ӷ���ʽΪ��PO43-+H2O?HPO42-+OH-��
��K1=7.5��10-3��K2=6.2��10-8��K3=2.2��10-13��NaH2PO4��Һ�з�������H2PO4-��ˮ�ⳣ������H2PO4-�ĵ���̶ȱȽϷ����ж���Һ����ԣ�Kh��H2PO4-��=$\frac{c��{H}_{3}P{O}_{4}��c��O{H}^{-}��}{{c��H}_{2}P{{O}_{4}}^{-}��}$=$\frac{Kw}{{K}_{1}}$=$\frac{1��1{0}^{-14}}{7.5��1{0}^{-3}}$=1.33��10-10��K2��H2PO4-�ĵ���ǿ��ˮ�⣬��Һ��c��H+����c��OH-������Һ�����ԣ�
�ʴ�Ϊ���ᣬKh��H2PO4-��=$\frac{c��{H}_{3}P{O}_{4}��c��O{H}^{-}��}{{c��H}_{2}P{{O}_{4}}^{-}��}$=$\frac{Kw}{{K}_{1}}$=$\frac{1��1{0}^{-14}}{7.5��1{0}^{-3}}$=1.33��10-10��K2��H2PO4-�ĵ���ǿ��ˮ�⣬��Һ��c��H+����c��OH-������Һ�����ԣ�
���� ���⿼�������漣���������ʵķ����жϣ���Ҫ��������ԭ��Ӧ����ת�ƺͻ�ѧ����ʽ��д��������ʵ���ƽ�⣬����ˮ��ƽ�������Ӧ�ã����ջ����ǹؼ�����Ŀ�ѶȽϴ�

 ���µ�������ʾ���������������л�����β��ռ22.2%��ȼúռ16.7%���ﳾռ16.3%����ҵռ15.7%���ݷ���������Ҫ��������Դ��ͼ��ʾ�����д�ʩ�����ܸ��ƿ����������ǣ�������
���µ�������ʾ���������������л�����β��ռ22.2%��ȼúռ16.7%���ﳾռ16.3%����ҵռ15.7%���ݷ���������Ҫ��������Դ��ͼ��ʾ�����д�ʩ�����ܸ��ƿ����������ǣ�������| A�� | Ӧ�ø�Ч�ྻ����Դת���������ڴ���ȼú��¯�ϰ�װ�͵�ȼ���������������������� | |
| B�� | �����̻���𣬼�ǿ��·�ﳾ��Ⱦ���ƣ�ǿ��·�汣�࣬��ˮ���� | |
| C�� | �����ͳ��������������糧�ȣ��ƹ��ʹ������������������������ŷ� | |
| D�� | �о���ú�������¼������������ͣ����͵���Դ�Ĺ�Ӧ�����ᳫ�������������ִ��������������� |
| A�� | 3 | B�� | 4 | C�� | 5 | D�� | 6 |
| A�� | pH=4���Ȼ����Һ��������ˮϡ��10������Һ��pH=5 | |
| B�� | pH=3��������pH=3��CH3COOH��Һ��ȣ�c��Cl-����C��CH3COO-�� | |
| C�� | pH=2��һԪ��HA��Һ��pH=12��NaOH��Һ�������1��1��Ϻ�c��Na+����c��A-�� | |
| D�� | pH��ͬ��NaOH��NaClO������Һ��ˮ�ĵ���̶���ͬ |
| ��Է������� | �е㣨�棩 | |
| �Ҵ� | 46 | 78.5 |
| ���� | 60 | 117.9 |
| �������� | 88 | 77.1 |
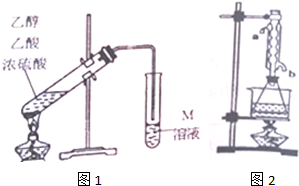
��1����ͼ1��ȡװ����Ҫ�������Ƭ���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������C������ȷѡ����ĸ����
A���������� B�������Ӳ� C����ȴ��Ӳ� D������Ӳ�
��2��M��ҺΪNa2CO3���ѧʽ����Һ��װ����ͨ�����ĵ���Ҫ���ڱ���M��Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹM��Һ��������ɵ�����ԭ����������Ҵ���������Na2CO3��Һ��
��3����ͬѧ��Ϊ�Ľ�ͼ1����ȡװ������������������ʣ�д��һ���Ľ����飺��װˮ�����ܣ������ȴЧ������Բ����ƿ��������ƿ������װ�¶ȼƿ��Ʒ�Ӧ�¶ȣ�
��4��ͼ1װ����ȡ�����������л��������Ҵ������ᣮͨ������ʵ��ɷ������������ĺ����������������£�
��ȷ����20.0g���������ֲ�Ʒ����ƿ�У���0.50mol•L-1NaOH�ζ�����̪��ָʾ�������յ�ʱ����NaOH��Һ�����Ϊ40.0mL
����ȡ20.0g���������ֲ�Ʒ��250mL��ƿ�У�����100mL2.1mol•L-1NaOH��Һ��Ͼ��Ⱥ�װ�������ܣ���ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ2��ʾ��������ȴ����0.50mol•L-1HCl�ζ�������NaOH���յ�ʱ������������Ϊ20.0mL��ʵ�������ˮ�������ܵ�b��ѡ��a��b���ܿ�ͨ�룮����ʵ��١��ڲ��������ݼ���ֲ�Ʒ��������������������Ϊ79.2%��
| A�� | ��ѧ��Ӧ�����DZ�ʾ��ѧ��Ӧ������������ | |
| B�� | һ������£������¶��ܼӿ컯ѧ��Ӧ���� | |
| C�� | ���淴Ӧ�ﵽ��ѧ��Ӧ��ʱ����Ӧ�;�ֹ������ | |
| D�� | ���淴Ӧ�ﵽ��ѧ��Ӧ��ʱ������Ӧ���ʵ����淴Ӧ���� |

 �����ȩ���ӽṹ����6�ֲ�ͬ��ѧ��������ԭ�ӣ�
�����ȩ���ӽṹ����6�ֲ�ͬ��ѧ��������ԭ�ӣ� ��Bת��Ϊ���ȩ�ķ�Ӧ����ΪCu/Ag���ȣ�
��Bת��Ϊ���ȩ�ķ�Ӧ����ΪCu/Ag���ȣ� ��
�� ��
��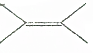 �Ķ��ȴ�����4�֣�
�Ķ��ȴ�����4�֣�